Difference between revisions of "Part:BBa K404117"
| Line 23: | Line 23: | ||
|} | |} | ||
<br /> | <br /> | ||
| − | <p style="text-align:justify;"> | + | <p style="text-align:justify;"> |
| − | + | <html><img src="https://static.igem.org/mediawiki/parts/0/0e/Freiburg10_Vectorplasmid_precursors_4.png" style="margin: 0px 20px 0px 0px; width: 500px;" /> | |
| − | + | ||
| − | + | ||
| − | <html><img src="https://static.igem.org/mediawiki/parts/0/0e/Freiburg10_Vectorplasmid_precursors_4.png" style="margin: 0px 20px 0px 0px; width: | + | |
</html> | </html> | ||
</p> | </p> | ||
<br /> | <br /> | ||
| + | <html> | ||
| + | <head> | ||
| + | <meta http-equiv="Content-Type" | ||
| + | content="text/html; charset=windows-1252"> | ||
| + | <meta name="Generator" | ||
| + | content="Microsoft Word 14 (filtered)"> | ||
| + | <style> | ||
| + | <!-- | ||
| + | /* Font Definitions */ | ||
| + | @font-face | ||
| + | {font-family:Cambria; | ||
| + | panose-1:2 4 5 3 5 4 6 3 2 4;} | ||
| + | @font-face | ||
| + | {font-family:Calibri; | ||
| + | panose-1:2 15 5 2 2 2 4 3 2 4;} | ||
| + | /* Style Definitions */ | ||
| + | p.MsoNormal, li.MsoNormal, div.MsoNormal | ||
| + | {margin-top:0cm; | ||
| + | margin-right:0cm; | ||
| + | margin-bottom:10.0pt; | ||
| + | margin-left:0cm; | ||
| + | line-height:115%; | ||
| + | font-size:11.0pt; | ||
| + | font-family:"Calibri","sans-serif";} | ||
| + | h1 | ||
| + | {mso-style-link:"Heading 1 Char"; | ||
| + | margin-top:24.0pt; | ||
| + | margin-right:0cm; | ||
| + | margin-bottom:0cm; | ||
| + | margin-left:0cm; | ||
| + | margin-bottom:.0001pt; | ||
| + | line-height:115%; | ||
| + | page-break-after:avoid; | ||
| + | font-size:14.0pt; | ||
| + | font-family:"Cambria","serif"; | ||
| + | color:red;} | ||
| + | p.MsoListParagraph, li.MsoListParagraph, div.MsoListParagraph | ||
| + | {margin-top:0cm; | ||
| + | margin-right:0cm; | ||
| + | margin-bottom:10.0pt; | ||
| + | margin-left:36.0pt; | ||
| + | line-height:115%; | ||
| + | font-size:11.0pt; | ||
| + | font-family:"Calibri","sans-serif";} | ||
| + | p.MsoListParagraphCxSpFirst, li.MsoListParagraphCxSpFirst, div.MsoListParagraphCxSpFirst | ||
| + | {margin-top:0cm; | ||
| + | margin-right:0cm; | ||
| + | margin-bottom:0cm; | ||
| + | margin-left:36.0pt; | ||
| + | margin-bottom:.0001pt; | ||
| + | line-height:115%; | ||
| + | font-size:11.0pt; | ||
| + | font-family:"Calibri","sans-serif";} | ||
| + | p.MsoListParagraphCxSpMiddle, li.MsoListParagraphCxSpMiddle, div.MsoListParagraphCxSpMiddle | ||
| + | {margin-top:0cm; | ||
| + | margin-right:0cm; | ||
| + | margin-bottom:0cm; | ||
| + | margin-left:36.0pt; | ||
| + | margin-bottom:.0001pt; | ||
| + | line-height:115%; | ||
| + | font-size:11.0pt; | ||
| + | font-family:"Calibri","sans-serif";} | ||
| + | p.MsoListParagraphCxSpLast, li.MsoListParagraphCxSpLast, div.MsoListParagraphCxSpLast | ||
| + | {margin-top:0cm; | ||
| + | margin-right:0cm; | ||
| + | margin-bottom:10.0pt; | ||
| + | margin-left:36.0pt; | ||
| + | line-height:115%; | ||
| + | font-size:11.0pt; | ||
| + | font-family:"Calibri","sans-serif";} | ||
| + | span.Heading1Char | ||
| + | {mso-style-name:"Heading 1 Char"; | ||
| + | mso-style-link:"Heading 1"; | ||
| + | font-family:"Cambria","serif"; | ||
| + | color:red; | ||
| + | font-weight:bold;} | ||
| + | .MsoChpDefault | ||
| + | {font-family:"Calibri","sans-serif";} | ||
| + | .MsoPapDefault | ||
| + | {margin-bottom:10.0pt; | ||
| + | line-height:115%;} | ||
| + | @page WordSection1 | ||
| + | {size:612.0pt 792.0pt; | ||
| + | margin:70.85pt 70.85pt 2.0cm 70.85pt;} | ||
| + | div.WordSection1 | ||
| + | {page:WordSection1;} | ||
| + | --> | ||
| + | </style> | ||
| + | </head> | ||
| + | <body lang="EN-US"> | ||
| + | <div class="WordSection1"> | ||
| + | <h1><span style="color: windowtext;">Part: | ||
| + | [AAV2]-left-ITR_pCMV_betaglobin</span></h1> | ||
| + | <p class="MsoNormal"> </p> | ||
| + | <p class="MsoNormal" style="text-align: justify;"><span | ||
| + | style="font-size: 10pt; line-height: 115%;">Producing | ||
| + | recombinant virus particles for therapeutical | ||
| + | applications is, besides specific cell targeting, purification and | ||
| + | quantification assays of AAV-2, one intention of the Virus Construction | ||
| + | Kit | ||
| + | provided by the iGEM team Freiburg_Bioware 2010. For obtaining a | ||
| + | modular | ||
| + | toolkit, the complex biological system of the Adeno-associated virus | ||
| + | serotype 2 | ||
| + | was examined by an exhaustive literature search. Subsequently, the | ||
| + | essential | ||
| + | components for AAV-2 particle production were extracted and redesigned | ||
| + | to match | ||
| + | the iGEM standard.</span></p> | ||
| + | <p class="MsoNormal" style="text-align: justify;"><span | ||
| + | style="font-size: 10pt; line-height: 115%;">The provided | ||
| + | tripartite system is independent of a | ||
| + | superinfection of Adeno- or herpes simplex viruses since the | ||
| + | genes encoding | ||
| + | the required helper-proteins are co-transfected. Inside the eukaryotic | ||
| + | host | ||
| + | cell, the DNA sequence containing the inverted terminal repeats (ITRs) | ||
| + | is | ||
| + | extracted and later encapsidated into the preformed capsids after | ||
| + | production of | ||
| + | single-stranded DNA. Consequently, this plasmid is known as the vector | ||
| + | plasmid | ||
| + | (pGOI). Promoter, <i>beta-globin</i> intron and the hGH | ||
| + | terminator signal are | ||
| + | flanked by the ITRs (ITRs, BBa_K404100 and BBa_K404101) and regulate | ||
| + | transgene | ||
| + | expression. The vector plasmid containing the desired gene of interest | ||
| + | is | ||
| + | cotransfected with the RepCap plasmid (BBa_K404001, BBa_K404002 or | ||
| + | BBa_K404003) | ||
| + | and the pHelper plasmid. To obtain the fully assembled vector plasmid, | ||
| + | several | ||
| + | assembly steps have to be performed. </span></p> | ||
| + | <p class="MsoNormal" style="text-align: justify;"><span | ||
| + | style="font-size: 10pt; line-height: 115%;">Facilitating the | ||
| + | cloning steps the iGEM team Freiburg_Bioware | ||
| + | 2010 provides the 5´nucleotide components in respect to the desired | ||
| + | gene of | ||
| + | interest, which are the left inverted terminal repeat (</span><span | ||
| + | style="font-size: 10pt; line-height: 115%;">BBa_K404100</span><span | ||
| + | style="font-size: 10pt; line-height: 115%;">) followed by | ||
| + | the CMV promoter (</span><span | ||
| + | style="font-size: 10pt; line-height: 115%;">BBa_K404102) and | ||
| + | a putative | ||
| + | enhancer-element (BBa_K404107). Compared to the parent construct | ||
| + | (BBa_K404114), | ||
| + | the presence of the beta-globin intron may enhance the expression of | ||
| + | the | ||
| + | downstream transgene. In context of the viral particle assembly, the | ||
| + | presence | ||
| + | of this genetic element may lower the packaging efficiency since the | ||
| + | latter is | ||
| + | affected by the size of the gene of interest encapsidated into the | ||
| + | virus | ||
| + | particle. Consequently, fewer functional particles are produced by the | ||
| + | eukaryotic host cell.</span></p> | ||
| + | <p class="MsoNormal"> </p> | ||
| + | </div> | ||
| + | </body> | ||
| + | </html> | ||
| + | |||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
Latest revision as of 12:18, 27 October 2010
[AAV2]-left-ITR_pCMV_betaglobin
| [AAV2-left-ITR_pCMV_betaglobin] | |
|---|---|
| |
| BioBrick Nr. | BBa_K404117 |
| RFC standard | RFC 10 |
| Requirement | pSB1C3 |
| Source | pAAV_MCS provided by Stratagene |
| Submitted by | [http://2010.igem.org/Team:Freiburg_Bioware FreiGEM 2010] |

Part: [AAV2]-left-ITR_pCMV_betaglobin
Producing recombinant virus particles for therapeutical applications is, besides specific cell targeting, purification and quantification assays of AAV-2, one intention of the Virus Construction Kit provided by the iGEM team Freiburg_Bioware 2010. For obtaining a modular toolkit, the complex biological system of the Adeno-associated virus serotype 2 was examined by an exhaustive literature search. Subsequently, the essential components for AAV-2 particle production were extracted and redesigned to match the iGEM standard.
The provided tripartite system is independent of a superinfection of Adeno- or herpes simplex viruses since the genes encoding the required helper-proteins are co-transfected. Inside the eukaryotic host cell, the DNA sequence containing the inverted terminal repeats (ITRs) is extracted and later encapsidated into the preformed capsids after production of single-stranded DNA. Consequently, this plasmid is known as the vector plasmid (pGOI). Promoter, beta-globin intron and the hGH terminator signal are flanked by the ITRs (ITRs, BBa_K404100 and BBa_K404101) and regulate transgene expression. The vector plasmid containing the desired gene of interest is cotransfected with the RepCap plasmid (BBa_K404001, BBa_K404002 or BBa_K404003) and the pHelper plasmid. To obtain the fully assembled vector plasmid, several assembly steps have to be performed.
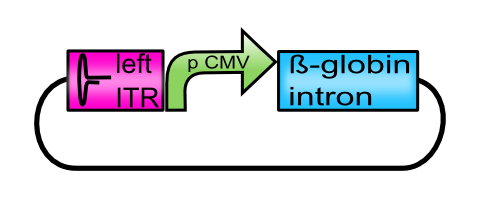
Facilitating the cloning steps the iGEM team Freiburg_Bioware 2010 provides the 5´nucleotide components in respect to the desired gene of interest, which are the left inverted terminal repeat (BBa_K404100) followed by the CMV promoter (BBa_K404102) and a putative enhancer-element (BBa_K404107). Compared to the parent construct (BBa_K404114), the presence of the beta-globin intron may enhance the expression of the downstream transgene. In context of the viral particle assembly, the presence of this genetic element may lower the packaging efficiency since the latter is affected by the size of the gene of interest encapsidated into the virus particle. Consequently, fewer functional particles are produced by the eukaryotic host cell.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
