Difference between revisions of "Part:BBa K4236002:Experience"
| Line 12: | Line 12: | ||
5. Centrifuged the bacterial solution at 12000 g, the precipitation was mixed with RIPA as a lysis buffer. Added loading buffer to heat at 96℃ for 10 min, underwent SDS-PAGE and Coomassie brilliant blue staining for expression test. | 5. Centrifuged the bacterial solution at 12000 g, the precipitation was mixed with RIPA as a lysis buffer. Added loading buffer to heat at 96℃ for 10 min, underwent SDS-PAGE and Coomassie brilliant blue staining for expression test. | ||
According to our SDS-PAGE result, we could see a band at around 52.04kD, which is in line with the molecular weight of UGT. This band only occurred in IPTG group, which confirmed with our induction. Besides, UGT expression at 12 h was higher than that at 36 h. We proposed that this may be due to the large number of bacteria at 36 h and the high survival pressure, which is not conducive to protein expression. Taken together, we have successfully expressed UGT in E.coli. | According to our SDS-PAGE result, we could see a band at around 52.04kD, which is in line with the molecular weight of UGT. This band only occurred in IPTG group, which confirmed with our induction. Besides, UGT expression at 12 h was higher than that at 36 h. We proposed that this may be due to the large number of bacteria at 36 h and the high survival pressure, which is not conducive to protein expression. Taken together, we have successfully expressed UGT in E.coli. | ||
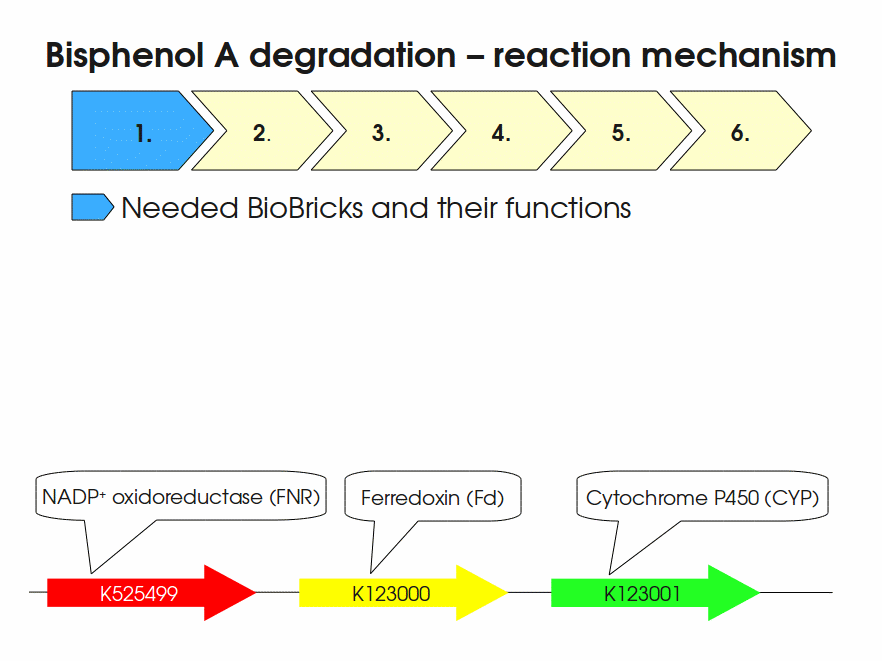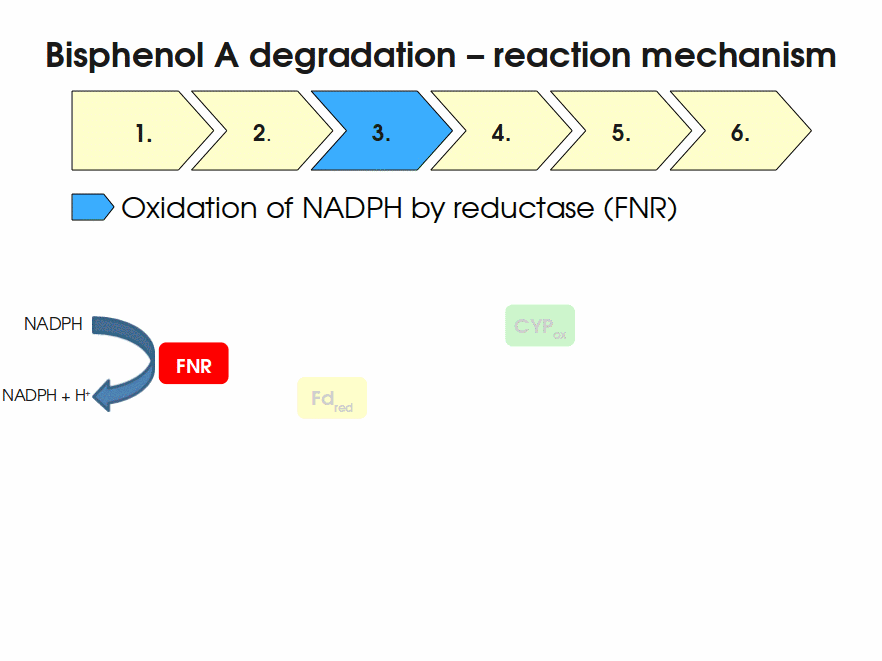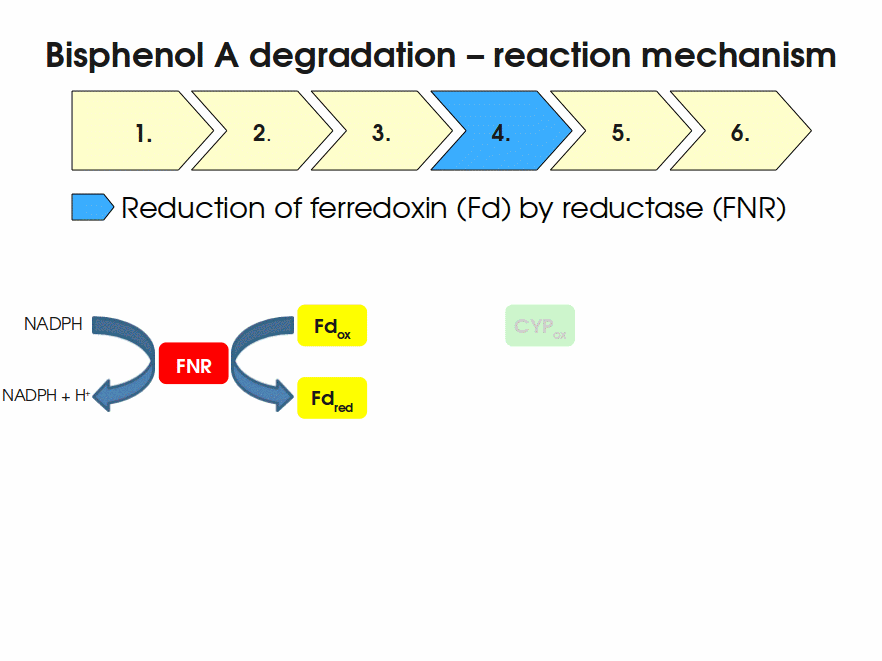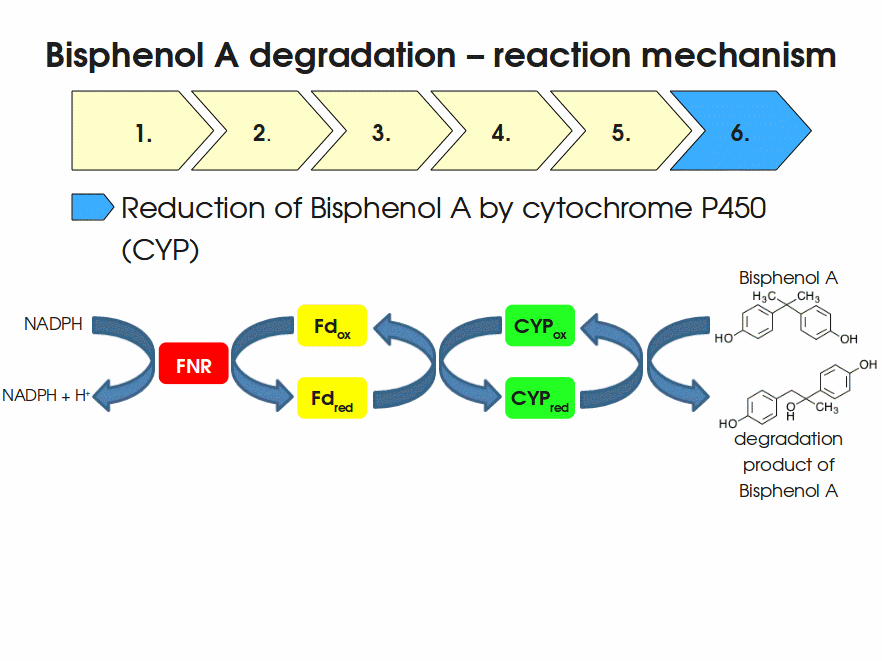
| + | [[Image:Bielefeld-Germany2011-BPAdegrad2.gif|center|700px|thumb|'''Fig. 1: Animation of proposed reaction mechanism of bisphenol A hydroxylation by the involved enzymes FNR (<partinfo>K525499</partinfo>), Fd (BisdA, <partinfo>K123000</partinfo>) and CYP (BisdB, <partinfo>K123001</partinfo>)''']] | ||
===User Reviews=== | ===User Reviews=== | ||
Revision as of 07:33, 8 October 2022
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_K4236002
Protocol we used: 1. Verification of the sequence. The sequence we submitted here was come from (物种). In order to get better field, we has done codon optimization for E.coli expression for this sequence. We contacted with a biology company to synthesize the sequence. 2. We constructed it into a EGFP tagged plasmid and transformed the plasmids into E. coli BL21(DE3). 3. After culture overnight, we picked a single colony added it into 4 ml LB medium with the corresponding antibiotic. The mix was then shook at 37℃ until OD = 600. 4. Dividing the liquid medium into 23 parts, adding 1 mM IPTG to two of them for inducing the expression 16℃ at 12 h or 36 h . The other one added nothing to serve as a control. 5. Centrifuged the bacterial solution at 12000 g, the precipitation was mixed with RIPA as a lysis buffer. Added loading buffer to heat at 96℃ for 10 min, underwent SDS-PAGE and Coomassie brilliant blue staining for expression test. According to our SDS-PAGE result, we could see a band at around 52.04kD, which is in line with the molecular weight of UGT. This band only occurred in IPTG group, which confirmed with our induction. Besides, UGT expression at 12 h was higher than that at 36 h. We proposed that this may be due to the large number of bacteria at 36 h and the high survival pressure, which is not conducive to protein expression. Taken together, we have successfully expressed UGT in E.coli.
User Reviews
UNIQe9b0e1433089833e-partinfo-00000003-QINU UNIQe9b0e1433089833e-partinfo-00000004-QINU
