Difference between revisions of "Part:BBa K3431048"
(→Introduction) |
|||
| Line 5: | Line 5: | ||
===Introduction=== | ===Introduction=== | ||
<html> | <html> | ||
| − | To develop an oral cancer detection device, we noticed that the pH conditions in human biological samples are able to affect the expression of reporter protein through influencing folding processes and changing protein | + | To develop an oral cancer detection device, we noticed that the pH conditions in human biological samples are able to affect the expression of reporter protein through influencing folding processes and changing protein structures<sup>1,2</sup>. Consequently, while we were finding a suitable expression protein for our detection device, we tested the effect of different pH conditions on mRFP (<a href="https://parts.igem.org/Part:BBa_E1010">BBa_E1010</a>), a monomeric red fluorescent protein firstly characterized by Robert E. Campbell et al.. In this experiment, we measured and observed the excitation and emission spectra of mRFP under six pH level, and described the appropriate working and measuring conditions of mRFP. |
</html> | </html> | ||
| − | |||
===Method=== | ===Method=== | ||
Revision as of 13:10, 26 October 2020
T7 promoter + RBS + mRFP + T7 terminator
Introduction
To develop an oral cancer detection device, we noticed that the pH conditions in human biological samples are able to affect the expression of reporter protein through influencing folding processes and changing protein structures1,2. Consequently, while we were finding a suitable expression protein for our detection device, we tested the effect of different pH conditions on mRFP (BBa_E1010), a monomeric red fluorescent protein firstly characterized by Robert E. Campbell et al.. In this experiment, we measured and observed the excitation and emission spectra of mRFP under six pH level, and described the appropriate working and measuring conditions of mRFP.
Method
We expressed mRFP through the addition of T7 terminator on an existing part BBa_K199118 so that this part can be expressed through our modified PURExpress in vitro protein synthesis kit (for further inquiries, please refer to our Measurement page). We reproduced the conditions of pH 2, pH 5, pH 7, pH8, pH 10, and pH12 by mixing different chemical compounds in double-distilled water. After expressing mRFP through the kit, we measured its excitation and emission spectra with a Synergy H1 microplate reader. We performed our data analysis and presentation through GraphPad Prism 5. For further interests, the procedures for this experiment can be found at our Protocol page.
Result
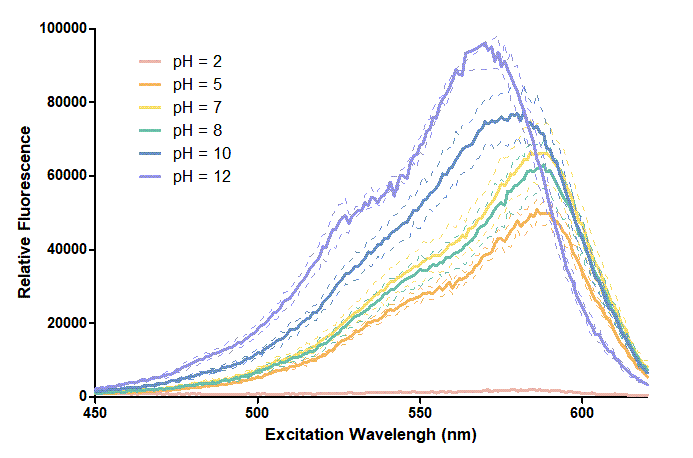
Figure 1. In this graph, we can find that when the pH value drops, the Fluorescent Intensity of mRFP also decreases correspondingly. In pH12, mRFP has the highest Fluorescent Intensity while it approaches zero when pH value becomes value 2. From our result, we could found that mRFP has stronger signals in alkaline environments, and the signal depletes over 50% once the pH value drops below 5.
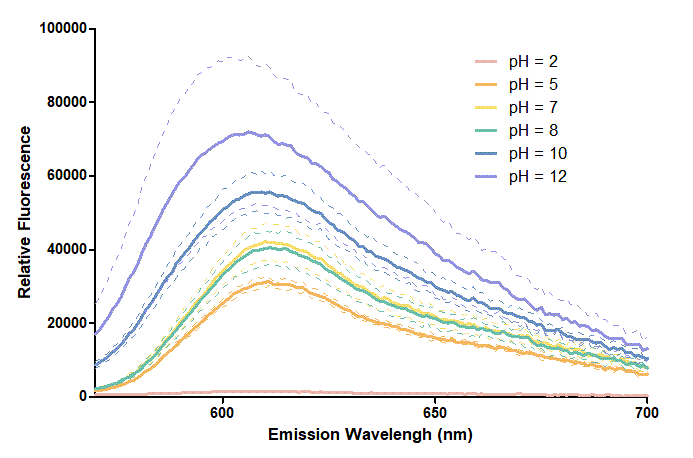
Figure 2. In the emission graph above, we can observe that the trend of fluorescent intensity is similar to the excitation graph. To the peak wavelength, we observe both the excitation graph and emission graph and point out their peak in different pH values. Regardless of pH2 since its intensity is quite weak, there is little difference between each pH value, only the absorbance wavelength of pH12 is lower than other pH values in both graph That is to say, if we want to use mRFP as our final product, we can make an alkaline environment to gain the most obvious results. However, we need to lower our emission and excitation wavelengths when we set up our plate reader.
Conclusion
Through performing excitation and emission spectroscopy on mRFP under different pH levels, we concluded that mRFP performs better while shifting its peak excitation wavelength toward a lower number under alkaline conditions. Since the biological samples for oral cancer detection mainly consist of environments that are not overly alkaline3, this characterisation of mRFP is not particularly useful in our project. Nevertheless, the result of our experiment shows that mRFP is more suitable for observation in alkaline conditions with a lower excitation wavelength to reach the best emission result for this fluorescence protein.
Reference
1. Battad, J. M., Wilmann, P. G., Olsen, S., Byres, E., Smith, S. C., Dove, S. G., Turcic, K. N., Devenish, R. J., Rossjohn, J., & Prescott, M. (2007). A structural basis for the pH-dependent increase in fluorescence efficiency of chromoproteins. Journal of molecular biology, 368(4), 998–1010. [1](https://doi.org/10.1016/j.jmb.2007.02.007)
2. T7 promoter + RBS + mRFP [2](https://parts.igem.org/Part:BBa_K199118)
3. mRFP[3](https://parts.igem.org/Part:BBa_E1010)
4. Johnson, D. E., Ai, H. W., Wong, P., Young, J. D., Campbell, R. E., & Casey, J. R. (2009). Red fluorescent protein pH biosensor to detect concentrative nucleoside transport. The Journal of biological chemistry, 284(31), 20499–20511. [4](https://doi.org/10.1074/jbc.M109.019042)
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 763
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 604
Illegal AgeI site found at 716 - 1000COMPATIBLE WITH RFC[1000]
