Difference between revisions of "Part:BBa J45992"
| Line 8: | Line 8: | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
Expression of ''osmY'' is dependent on σ<sup>S</sup> ''in vivo'' <cite>Hengge-Aronis-1993</cite>. Schellhorn ''et al.'' previously demonstrated that an ''osmY::lacZ'' fusion generated the highest transcriptional signal in stationary phase as compared to nine other σ<sup>S</sup>-dependent promoter-''lacZ'' fusions <cite>Schellhorn-1998, Vijayakumar-2004</cite>. In addition, the ''osmY::lacZ'' fusion generated only a small transcriptional signal during exponential growth. | Expression of ''osmY'' is dependent on σ<sup>S</sup> ''in vivo'' <cite>Hengge-Aronis-1993</cite>. Schellhorn ''et al.'' previously demonstrated that an ''osmY::lacZ'' fusion generated the highest transcriptional signal in stationary phase as compared to nine other σ<sup>S</sup>-dependent promoter-''lacZ'' fusions <cite>Schellhorn-1998, Vijayakumar-2004</cite>. In addition, the ''osmY::lacZ'' fusion generated only a small transcriptional signal during exponential growth. | ||
| + | |||
| + | ==Contribution: Macquarie University 2019== | ||
| + | '''Group:''' Macquarie_Australia | ||
| + | <br>'''Authors:''' Samuel Beach | ||
| + | <br>'''Summary:''' We compared the expression of our cyclic-di-GMP riboswitch + eGFP bioparts over 60 hours of incubation between this, a Lac (BBa_R0010), and a Tac (part #) promoter. All three promoters showed significant increase in expression starting at the stationary phase, however the Lac and Tac promoters showed a linear increase from that point while the Full-length stationary phase osmY promoter significantly increased expression then plateued after several hours. | ||
| + | |||
| + | <p>In order to characterise the mode of action of the riboswitch/promoter combinations, we collaborated with Team Sydney Australia to perform GFP fluorescence assays. These assays were used to quantify the production of eGFP between our reporter constructs, as well as comparing the function of the stationary phase promoter against two inducible promoters, Lac [BBa_R0010] and Tac <b>PART NUMBER</b> Samples were measured with the BMG Pherastar plate reader to measure eGFP (Ex485 nm Em520 nm) and OD (600 nm).</p> | ||
| + | |||
| + | <p> These results demonstrate that while the production of eGFP increases at 6-8 hours for all the promoters (i.e. roughly the point at which samples would be entering the stationary phase), the Lac <b>(Fig. 1 & 4)</b> and Tac <b>(Fig. 2 & 5)</b> inducible promoters show a steady, somewhat linear accumulation of eGFP over time, the stationary phase promoter <b>(Fig. 3)</b> shows a sharp increase and beings to level out after ~20 hours.</p> | ||
| + | |||
| + | <p> These results are consistent with previous characterisations of the promoter, which observed significant increases in β-galactosidase concentration after entry into the stationary phase at 5 hours (Hengge, Aronis: 1993) </p> | ||
| + | |||
| + | |||
| + | https://2019.igem.org/wiki/images/thumb/5/50/T--Macquarie_Australia--lac01.jpg/800px-T--Macquarie_Australia--lac01.jpg | ||
| + | <br><i>Figure 1: eGFP produced by cells transformed with the Lac riboswitch constructs [BBa_K3151028] over 60 hours.</i> | ||
| + | |||
| + | https://2019.igem.org/wiki/images/thumb/9/99/T--Macquarie_Australia--tac01.jpg/800px-T--Macquarie_Australia--tac01.jpg | ||
| + | <br><i>Figure 2: eGFP produced by cells transformed with the Tac riboswitch constructs <b>PART #</b> over 60 hours.</i> | ||
| + | |||
| + | https://2019.igem.org/wiki/images/thumb/0/06/T--Macquarie_Australia--stat01.jpg/800px-T--Macquarie_Australia--stat01.jpg | ||
| + | <br><i>Figure 3: eGFP produced by cells transformed with the Stationary phase riboswitch constructs [BBa_K3151011] over 60 hours.</i> | ||
| + | |||
| + | https://2019.igem.org/wiki/images/thumb/7/78/T--Macquarie_Australia--UsydLac01.jpg/546px-T--Macquarie_Australia--UsydLac01.jpg | ||
| + | <br><i>Figure 4: eGFP produced by cells transformed with the Lac riboswitch constructs [BBa_K3151028] over 30 hours. Data provided by Usyd iGEM.</i> | ||
| + | |||
| + | https://2019.igem.org/wiki/images/thumb/1/1c/T--Macquarie_Australia--UsydTac01.jpg/548px-T--Macquarie_Australia--UsydTac01.jpg | ||
| + | <br><i>Figure 5: eGFP produced by cells transformed with the Tac riboswitch constructs <b>PART #</b> over 30 hours. Data provided by Usyd iGEM.</i> | ||
| + | |||
| + | |||
| + | <br> | ||
<biblio> | <biblio> | ||
Revision as of 09:10, 21 October 2019
Full-length stationary phase osmY promoter
BBa_J45992 is a stationary phase promoter derived from the promoter that controls transcription of osmY in Escherichia coli Yim-1992, Yim-1994. BBa_J45992 is active in stationary phase and under high osmotic pressure conditions.
Usage and Biology
Expression of osmY is dependent on σS in vivo Hengge-Aronis-1993. Schellhorn et al. previously demonstrated that an osmY::lacZ fusion generated the highest transcriptional signal in stationary phase as compared to nine other σS-dependent promoter-lacZ fusions Schellhorn-1998, Vijayakumar-2004. In addition, the osmY::lacZ fusion generated only a small transcriptional signal during exponential growth.
Contribution: Macquarie University 2019
Group: Macquarie_Australia
Authors: Samuel Beach
Summary: We compared the expression of our cyclic-di-GMP riboswitch + eGFP bioparts over 60 hours of incubation between this, a Lac (BBa_R0010), and a Tac (part #) promoter. All three promoters showed significant increase in expression starting at the stationary phase, however the Lac and Tac promoters showed a linear increase from that point while the Full-length stationary phase osmY promoter significantly increased expression then plateued after several hours.
In order to characterise the mode of action of the riboswitch/promoter combinations, we collaborated with Team Sydney Australia to perform GFP fluorescence assays. These assays were used to quantify the production of eGFP between our reporter constructs, as well as comparing the function of the stationary phase promoter against two inducible promoters, Lac [BBa_R0010] and Tac PART NUMBER Samples were measured with the BMG Pherastar plate reader to measure eGFP (Ex485 nm Em520 nm) and OD (600 nm).
These results demonstrate that while the production of eGFP increases at 6-8 hours for all the promoters (i.e. roughly the point at which samples would be entering the stationary phase), the Lac (Fig. 1 & 4) and Tac (Fig. 2 & 5) inducible promoters show a steady, somewhat linear accumulation of eGFP over time, the stationary phase promoter (Fig. 3) shows a sharp increase and beings to level out after ~20 hours.
These results are consistent with previous characterisations of the promoter, which observed significant increases in β-galactosidase concentration after entry into the stationary phase at 5 hours (Hengge, Aronis: 1993)
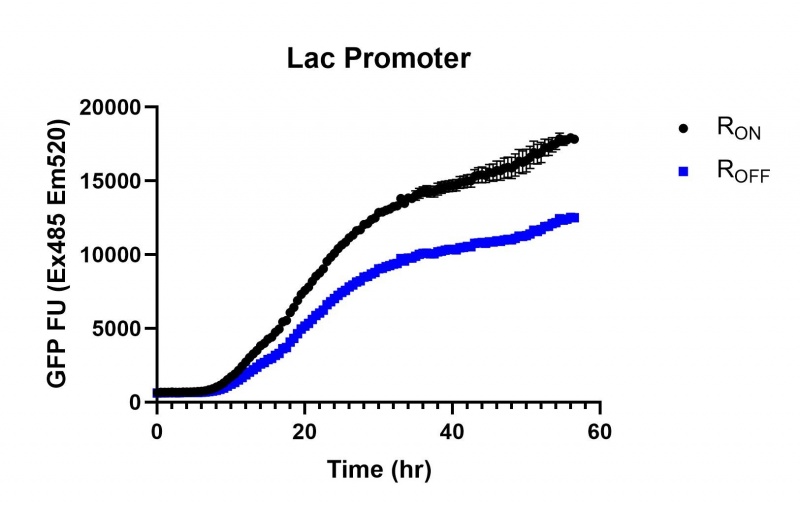
Figure 1: eGFP produced by cells transformed with the Lac riboswitch constructs [BBa_K3151028] over 60 hours.
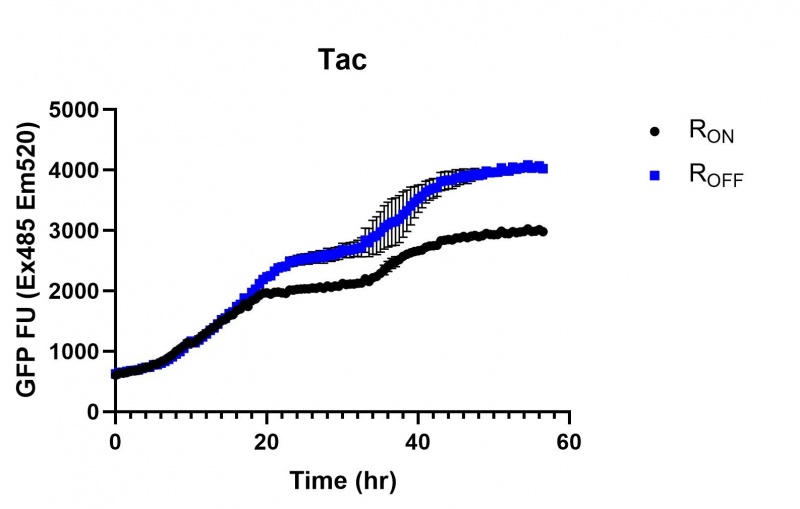
Figure 2: eGFP produced by cells transformed with the Tac riboswitch constructs PART # over 60 hours.
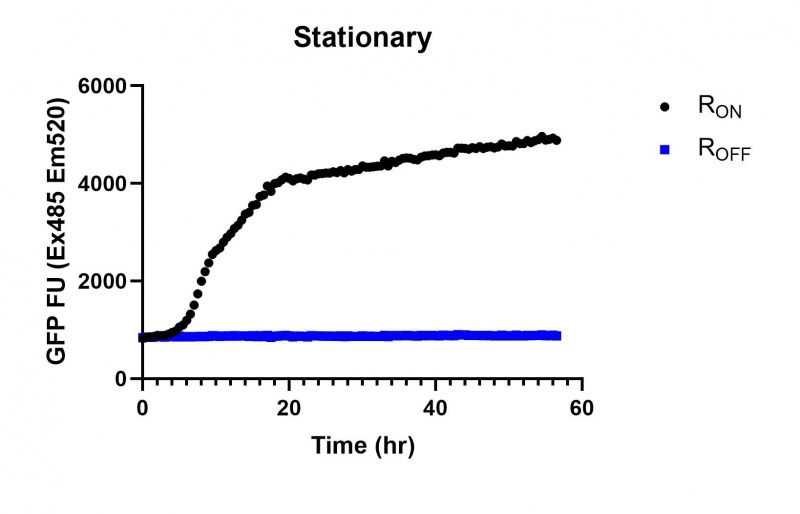
Figure 3: eGFP produced by cells transformed with the Stationary phase riboswitch constructs [BBa_K3151011] over 60 hours.
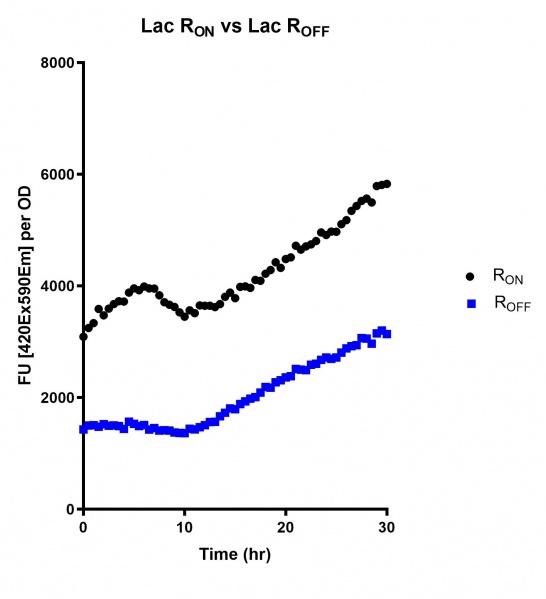
Figure 4: eGFP produced by cells transformed with the Lac riboswitch constructs [BBa_K3151028] over 30 hours. Data provided by Usyd iGEM.
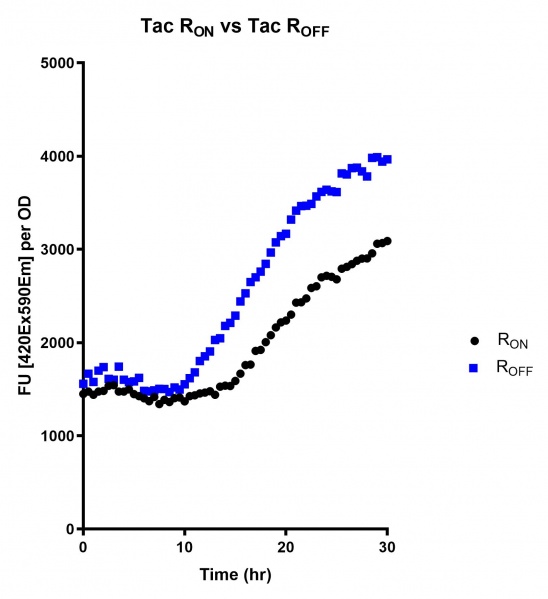
Figure 5: eGFP produced by cells transformed with the Tac riboswitch constructs PART # over 30 hours. Data provided by Usyd iGEM.
<biblio>
- Yim-1994 pmid=8282684
- Yim-1992 pmid=1317380
- Hengge-Aronis-1993 pmid=8416901
- Vijaykumar-2004 pmid=15576800
- Schellhorn-1998 pmid=9829938
</biblio>
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]