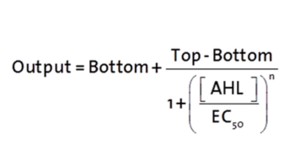Difference between revisions of "Part:BBa R0062:Experience"
(→Characterization of the pLuxR dose response to OHHL by plate reader analysis and single cell analysis) |
(→Characterization of the pLuxR dose response to OHHL by plate reader analysis and single cell analysis) |
||
| Line 24: | Line 24: | ||
===Characterization of the pLuxR dose response to OHHL by plate reader analysis and single cell analysis=== | ===Characterization of the pLuxR dose response to OHHL by plate reader analysis and single cell analysis=== | ||
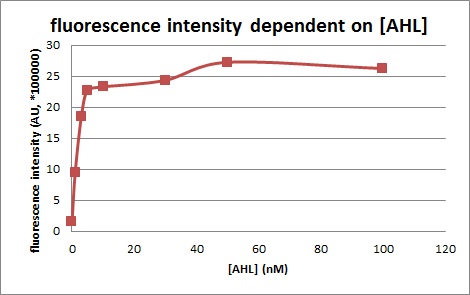
[[File:Wtg1platereader.jpg|left|400px|thumb|<b>Figure 1. Sensitivity curve of the wild type promoter</b> analyzed on the Tecan Infinity M200 microtiter plate reader. The OHHL concentration range is plotted against the standarized fluorescence (au).<br> EC<sub>50</sub>=5.86, R<sub>2</sub>= 0.87,n=1.7<br>]] | [[File:Wtg1platereader.jpg|left|400px|thumb|<b>Figure 1. Sensitivity curve of the wild type promoter</b> analyzed on the Tecan Infinity M200 microtiter plate reader. The OHHL concentration range is plotted against the standarized fluorescence (au).<br> EC<sub>50</sub>=5.86, R<sub>2</sub>= 0.87,n=1.7<br>]] | ||
| − | <b> Primary characterization of the dose reponse curve by microtiter plate analysis</b><br><br>We cloned the promoter BBa_R0062 in the BBa_J09855 construct and cloned a GFP gene as reporter. The GFP part allows us to make fluorescence measurement in microtiter plate experiment as well as in single cell analysis to characterize the promoter depending on [OHHL], by recording the fluorescence [au]. According to literature | + | <b> Primary characterization of the dose reponse curve by microtiter plate analysis</b> |
| − | [http://www.ncbi.nlm.nih.gov/pubmed/18760602 (Geske G.D. Evaluation of a focused library of N-aryl L-homoserine lactones reveals a new set of potent quorum sensing modulators)] we could define the first experimental OHHL ranges to induce the promoter. After some fine tunning and adjusting experiment we could narrow down the ranges to obtain a dose response curve in a reasonable resolution for the BBa_R0062 promoter to the OHHL (3-oxo-hexanoyl-l-homoserine-lactone). See Figure 1.<br> | + | <h1>Fluorescence data analysis</h1> |
| + | [[File:300-Formulapluxr1.png|left|300px]] | ||
| + | <p>The fitting of the following graphs was performed using this equation :<br><br> | ||
| + | Output = eGFP levels [au]<br> | ||
| + | Top = maximal eGFP level [au]("full induction")<br> | ||
| + | Bottom = minimal eGFP level [au](“leakiness”)<br> | ||
| + | n = Hill coefficient (“cooperativity”)<br> | ||
| + | EC<sub>50</sub> = Half-maximal effective concentration (“sensitivity”)<br> | ||
| + | [AHL]=OHHL concentration [nM]</p> | ||
| + | <br clear="all"/> | ||
| + | <br><br>We cloned the promoter BBa_R0062 in the BBa_J09855 construct and cloned a GFP gene as reporter. The GFP part allows us to make fluorescence measurement in microtiter plate experiment as well as in single cell analysis to characterize the promoter depending on [OHHL], by recording the fluorescence [au]. According to literature | ||
| + | [http://www.ncbi.nlm.nih.gov/pubmed/18760602 (Geske G.D. Evaluation of a focused library of N-aryl L-homoserine lactones reveals a new set of potent quorum sensing modulators)]we could define the first experimental OHHL ranges to induce the promoter. After some fine tunning and adjusting experiment we could narrow down the ranges to obtain a dose response curve in a reasonable resolution for the BBa_R0062 promoter to the OHHL (3-oxo-hexanoyl-l-homoserine-lactone). See Figure 1.<br> | ||
<br clear="all"/> | <br clear="all"/> | ||
<b>Single cell characterization of the dose response in liquid culture and on agar plate</b> <br><br> | <b>Single cell characterization of the dose response in liquid culture and on agar plate</b> <br><br> | ||
Revision as of 22:19, 4 October 2013
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
The team from Davidson College and Missouri Western State University discovered that this part promotes "backwards transcription" when LuxR protein is present and AHL-3OC6 is absent. You can read [http://www.ibc7.org/article/journal_v.php?sid=265 the paper that documents this unexpected "backwards promoter activity"] in their open access paper.
Applications of BBa_R0062
User Reviews
UNIQ52214909c08daef1-partinfo-00000000-QINU UNIQ52214909c08daef1-partinfo-00000001-QINU No review score entered. ETH Zurich 2013
Characterization of the pLuxR dose response to OHHL by plate reader analysis and single cell analysis
Primary characterization of the dose reponse curve by microtiter plate analysis
Fluorescence data analysis
The fitting of the following graphs was performed using this equation :
Output = eGFP levels [au]
Top = maximal eGFP level [au]("full induction")
Bottom = minimal eGFP level [au](“leakiness”)
n = Hill coefficient (“cooperativity”)
EC50 = Half-maximal effective concentration (“sensitivity”)
[AHL]=OHHL concentration [nM]
We cloned the promoter BBa_R0062 in the BBa_J09855 construct and cloned a GFP gene as reporter. The GFP part allows us to make fluorescence measurement in microtiter plate experiment as well as in single cell analysis to characterize the promoter depending on [OHHL], by recording the fluorescence [au]. According to literature
[http://www.ncbi.nlm.nih.gov/pubmed/18760602 (Geske G.D. Evaluation of a focused library of N-aryl L-homoserine lactones reveals a new set of potent quorum sensing modulators)]we could define the first experimental OHHL ranges to induce the promoter. After some fine tunning and adjusting experiment we could narrow down the ranges to obtain a dose response curve in a reasonable resolution for the BBa_R0062 promoter to the OHHL (3-oxo-hexanoyl-l-homoserine-lactone). See Figure 1.
Single cell characterization of the dose response in liquid culture and on agar plate
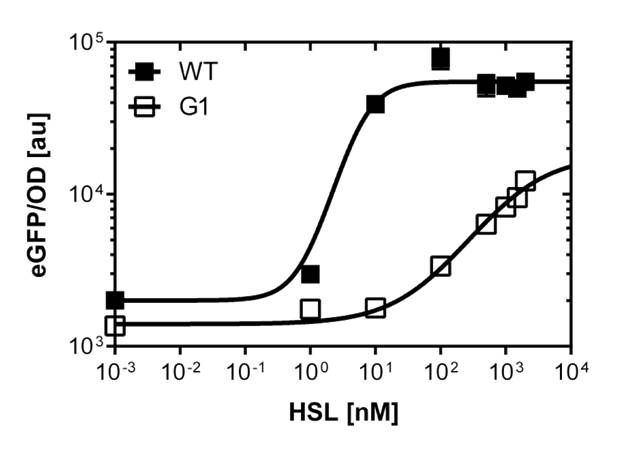
To obtain high quality data we did single cell analysis over the range defined above. Obviously the EC50 changed compared to the microtiter plate data.
See Figure 2.
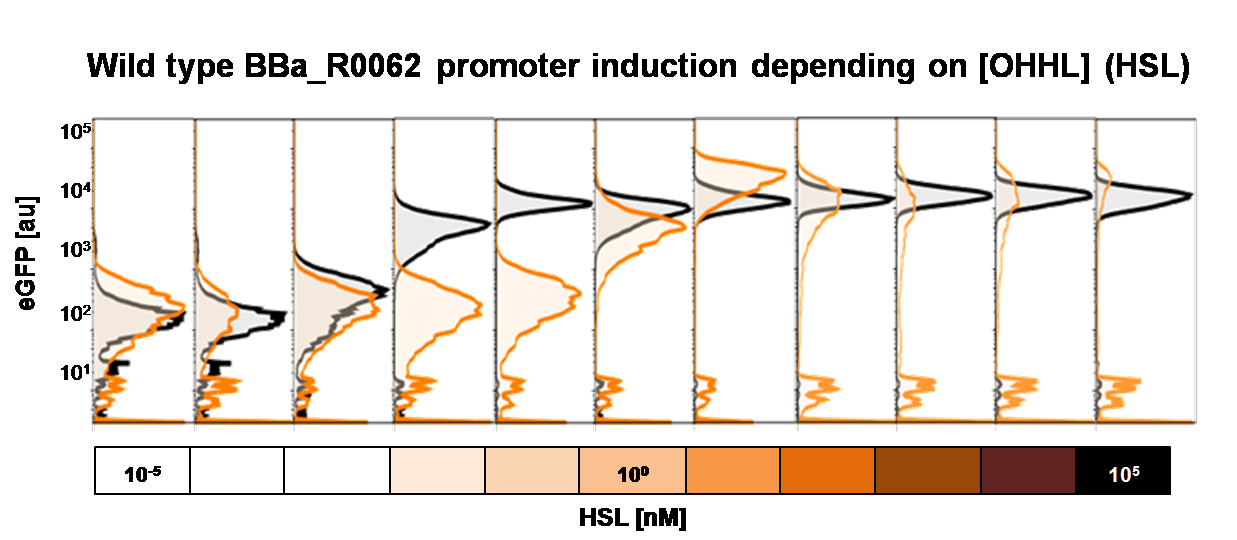
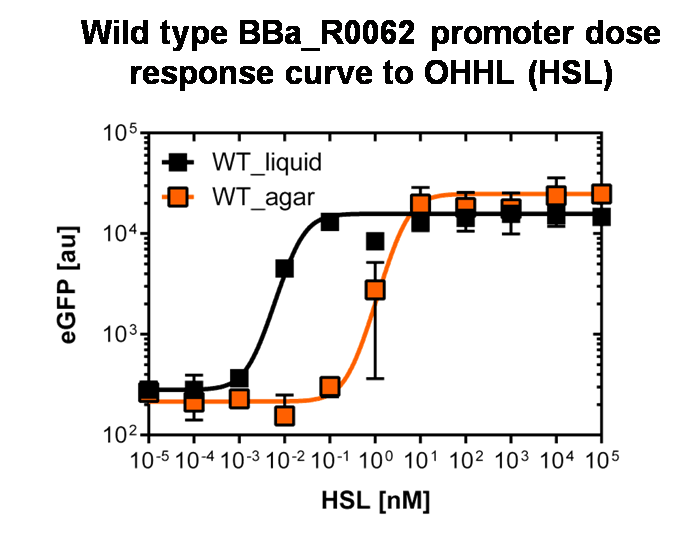
The black curve shows the response of to OHHL in liquid culture, the orange curve shows the response to OHHL on agar plates
To obtain high quality data we did single cell analysis over the dose response range defined above. Obviously the EC50 changed compared to the plate reader data. Figure 2. The plot shows the dose reponse curve of the promoter depending on AHL concentration. We performed this experiment in liquid culture as well as on agar plates (OHHL was added to the melted 1.5% agar), which was closer to our project. We observed a 220 time shift in the dose response between the liquid culture single cell analysis and the one in agar plates. See Figure 3 UNIQ52214909c08daef1-partinfo-00000003-QINU
|
•••••
SUN(Tsinghua) |
Part was sequenced and functional. |
|
•••••
iGEM12_OUC-China |
|
•••••
|
In order to optimize the function of the lux promoter, We creat a hybrid promoter BBa_K737067 which combines plux with OmpR, and in this case, plux can be led to expression by HSL and obtain by phosphorylation of OmpR protein.. According to the research of it before, we camp up with a program . Keep the sequencing of Lux Box, -35box and -10box., Then put the sequencing of C3 point of OmpR promoter into -35box and -10box.
Figure 1 structural representation of Plux promoter
Figure 2 the analysis of conserved sequence of pLux by Weblogo Plux promoter is organized from the promoter the shining gene of V. fischeri(R0062). Through analyzing common hybrid promoter of Lux by Weblogo , we found that the sequence of Lux Box is conserved. In benefit of LuxR Binding Site, -35box and -10box of the promoter the shining gene of V. fischeri(R0062).
Figure 3 : Our design of pLux-OmpR hybrid promoter We link the hybrid promoter to the test circuit. For control group experiment, we put strain A to LB medium adding 20% Sucrose solution and not adding any sucrose solution to cultivate in 37% shake flask , and detect the change of OD value and fluorescence value between 12h.
Though high concentration sucrose solution restrains the grain of E.coli, it doesn’t have effect on the work of circuit. In conclusion, we find that high osmotic pressure has no effect on quorum sensing circuit and the activated of plux promoter. Strain A could serve as control group of pLux-OmpR hybrid promoter. |
Measurement of pLux/OmpR hybrid promoter's expression property
Place strain B in 100 mL LB medium with gradient osmotic pressure stress and culture in shake-flask in 37°C.Calculate RFU of each time point ,and we get a surprising result!

Figure 6 Experimental Group 1 Relative Fluorescence Unit--Time Scatter graph

From the figure 6 we can see that when stressed by 7% sucrose, GFP's expression is stronger than those cultured in normal condition.
However,when stressed by 14% sucrose, GFP's RFU is 3 times as high as normal culture condition.This indicates that sucrose stress promotes GFP's expression via gene circuit.
UNIQ52214909c08daef1-partinfo-00000007-QINU
XMU-China 2011
|
••••
|
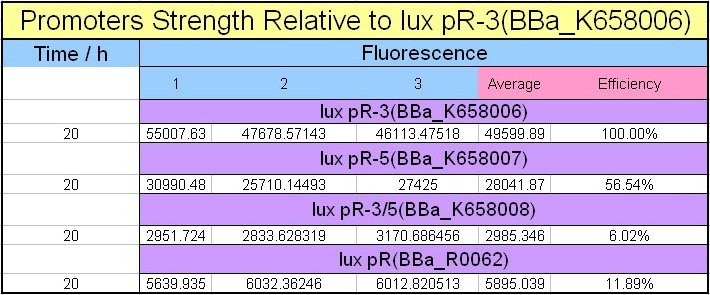
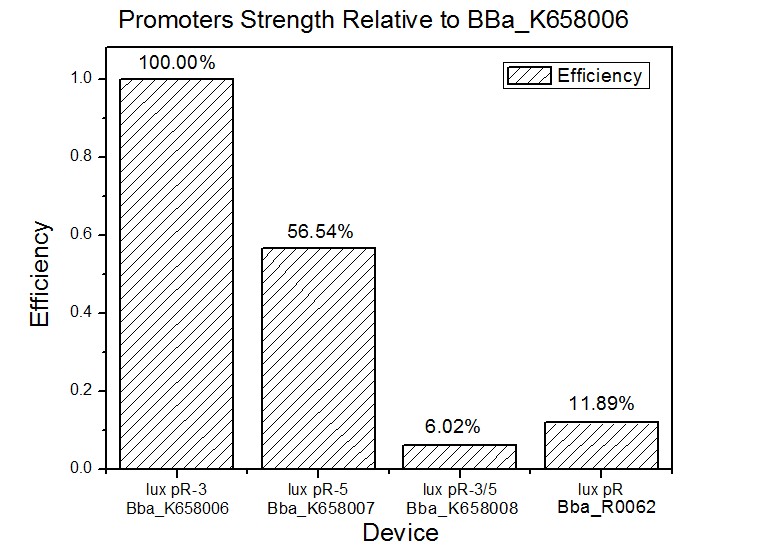
Site-directed mutagenesis at position 3,5 and 3/5 of BBa_R0062On the basis of the nucleotide sequence of the lux pR promoter, the 20 base pair inverted repeat ACCTGTAGGA TCGTACAGGT might consititude a protein binding site. And we also learned that mutagenesis at position 3 and position 5 might cause dramatic change on the expression of downstream gene. Therefore, we generated 3 mutants (BBa_K658006 BBa_K658007 BBa_K658008) of the promoter lux pR by site-directed mutagenesis at position 3, 5 and 3/5. By testing their strength in IR-GFP devices, we prove that mutagenesis at position 3 and position 5 can change the expression of downstream gene. Mutated promoters lux pR-3 (BBa_K658006) and lux pR-5 (BBa_K658007) dramatically enhanced the expression of the downstream gene compared with wild type promoter lux pR (R0062), while mutated promoter lux pR-3/5 (BBa_K658008) gave an even weaker expression of the downstream gene than promoter lux pR (R0062). Based on the information about the mutated promoters, we constructed a series of population-control devices which can maintain the cell density of bacteria population at several certain values. Lux pR strength testing deviceTo test the strength of promoters lux pR(BBa_R0062) and its 3 mutants lux pR-3 (BBa_K658006), lux pR-5(BBa_K658007), lux pR-3/5(BBa_K658008) , we constructed four devices( BBa_K658016 BBa_K658017 BBa_K658018 BBa_K658019). If promoter lacl+pL(BBa_R0011) is induced by isopropyl-b-D-thiogalactopyranoside (IPTG), this device will be switched on. At sufficiently high cell density, this device produces greenish tint visible by naked eye. By measuring florescent intensities at steady state of the cell growth for these four IR-GFP devices, the strength of a promoter lux pR could be defined.
The results are shown in following figures:
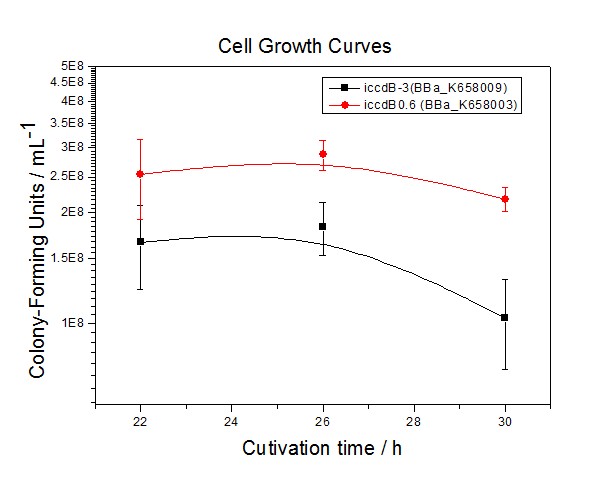
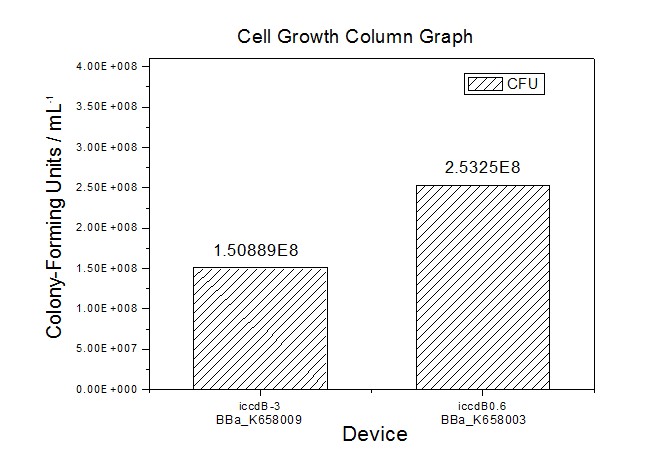
Application----a series of population-control devicesThe study of the mutated promoters lux pR-3 (BBa_K658006), lux pR-5(BBa_K658007) and lux pR-3/5(BBa_K658008) can be applied to construct a series of population-control devices based on iccdB0.6 (BBa_K658003). These devices—iccdB0.6(BBa_K658003), iccdB-3(BBa_K658009), iccdB-5(BBa_K658010) and iccdB-3/5(BBa_K658011) program the steady-state cell density maintaining at different levels.
The results are shown in following figures:
This matched the result of the test on four lux pR promoters’ strength in our IR-GFP device (BBa_K658016) mentioned above. As is shown in figure 2, promoter lux pR-3 has the highest strength of the four. It is probable that mutation at position 3 lowers the threshold for the binding reaction between LuxR/AHL protein complex and promoter lux pR, which starts the Quorum Sensing system at a relatively earlier period with a lower cell density compared with circuits regulated by wild type promoter lux pR (BBa_R0062). Once the QS system is started, downstream killer protein expresses. The viable cell density reaches a steady state when cell growth rate equals to its death rate. Generally, steady-state cell density seems to fluctuate at the cell density when QS is started. Thus, the higher strength a promoter has, the earlier the population-control device is started, leading to a lower steady-state cell density.
|
|
No review score entered. iGEM Tokyo_Tech 2010 |
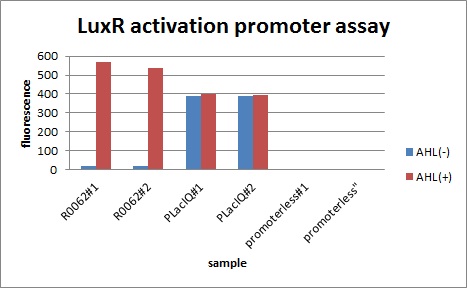
In order to characterize R0062, Plux repression promoter, we constructed K395100 combining R0062 and K121013, which is a promoter-less gfp reporter (rbs-gfp-ter-ter) on pSB6A1 and used a fusion of PlacIq (I14032) to gfp (K121013) as a positive control and used promoterless gfp (K121013) as a negative control.
|
|
Antiquity |
This review comes from the old result system and indicates that this part did not work in some test. |
|
•••••
wmholtz |
Using this part, I have successfully constructed and tested a quorum sensing circuit in E. coli. |
|
•••
Aberdeen_Scotland 2009 |
Our miniprep, digest and gel gave expected results. However we did not use this part for our cloning. |
|
No review score entered. NYMU-Taipei 2009 |
|
UNIQ52214909c08daef1-partinfo-00000012-QINU