Difference between revisions of "Part:BBa K525224"
JSchwarzhans (Talk | contribs) (→Expression in E. coli) |
(→Identification and localisation) |
||
| Line 67: | Line 67: | ||
===Identification and localisation=== | ===Identification and localisation=== | ||
| − | After a cultivation time of 18 h the mRFP|CspB fusion protein has to be localized in ''E. coli'' KRX. Therefor a part of the produced biomass was mechanically disrupted and the resulting lysate was wahed with ddH<sub>2</sub>O. Then the lysate was treted with ionic, nonionic and zwitterionic detergents to release the | + | After a cultivation time of 18 h the mRFP|CspB fusion protein has to be localized in ''E. coli'' KRX. Therefor a part of the produced biomass was mechanically disrupted and the resulting lysate was wahed with ddH<sub>2</sub>O. Then the lysate was treted with ionic, nonionic and zwitterionic detergents to release the mRFP|CspB out of the membranes, if it intigrates. From the other part of the cells the periplasm was detached by using a osmotic shock. |
| − | The fluorescence in all cultivation fractions plus the fluorescence in the lysis und wash fraction shows that the fusion protein is water soluble and sediment | + | The fluorescence in all cultivation fractions plus the fluorescence in the lysis und wash fraction shows that the fusion protein is water soluble and doesn't sediment doesn't during centrifugation. Together with the flourescence in 4 of 5 of the detergent fractions indicate that a part of the produced proteins form inclusion bodies. |
In comparison with the mRFP fusion protein of ???, wich has a lipid anchor, a minor relative fluorescence per OD<sub>600</sub> in all cultivation and detergent fractions was detected (fig. 3). Together with the decreasing RFU/OD<sub>600</sub> after 14 h of cultivation (fig. 2) indicates this rusult a postive effect of the lipid anchor on the protein stability. | In comparison with the mRFP fusion protein of ???, wich has a lipid anchor, a minor relative fluorescence per OD<sub>600</sub> in all cultivation and detergent fractions was detected (fig. 3). Together with the decreasing RFU/OD<sub>600</sub> after 14 h of cultivation (fig. 2) indicates this rusult a postive effect of the lipid anchor on the protein stability. | ||
Revision as of 00:24, 22 September 2011
S-layer cspB from Corynebacterium halotolerans with TAT-sequence, PT7 and RBS
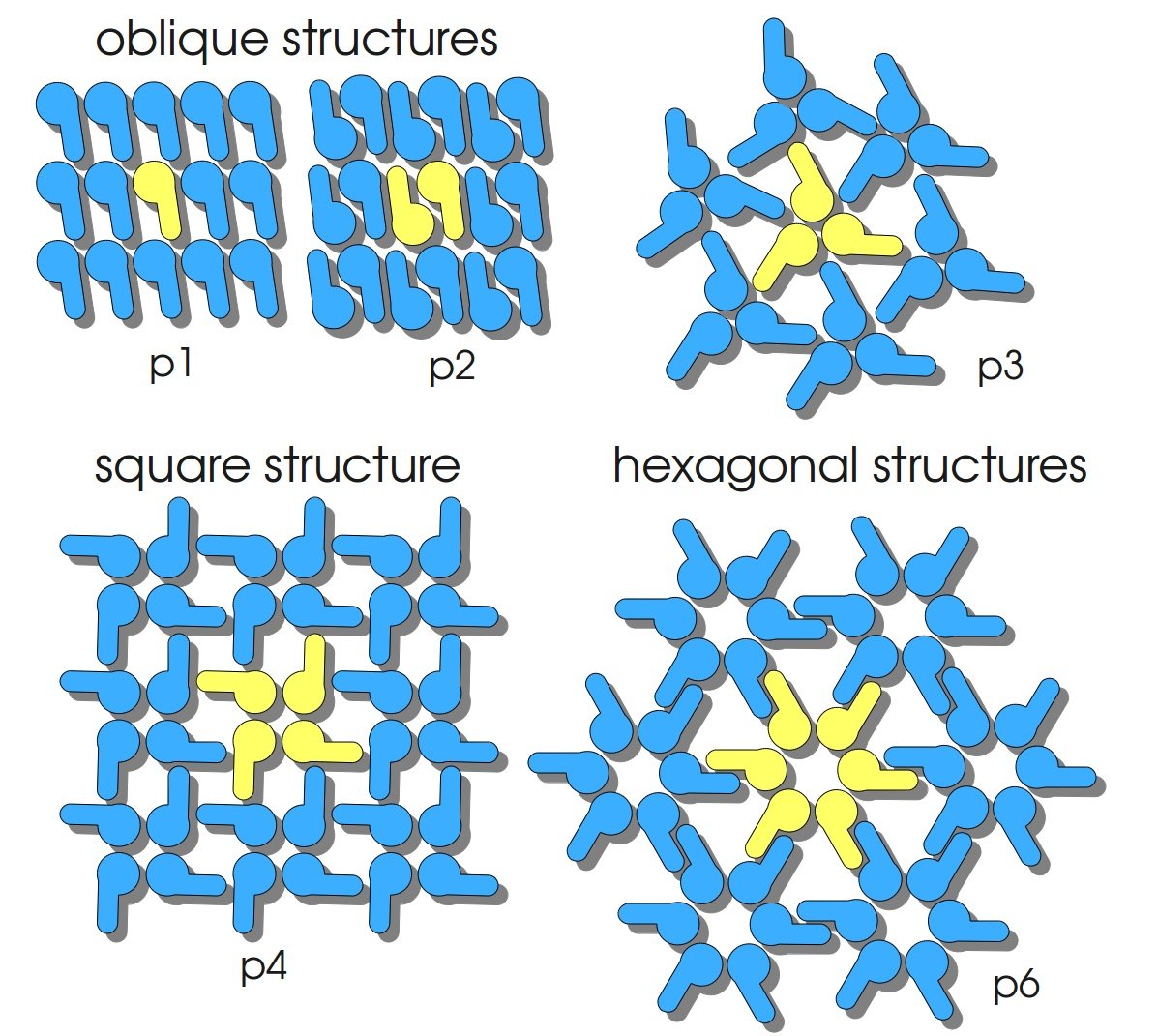
S-layers (crystalline bacterial surface layer) are crystal-like layers consisting of multiple protein monomers and can be found in various (archae-)bacteria. They constitute the outermost part of the cell wall. Especially their ability for self-assembly into distinct geometries is of scientific interest. At phase boundaries, in solutions and on a variety of surfaces they form different lattice structures. The geometry and arrangement is determined by the C-terminal self assembly-domain, which is specific for each S-layer protein. The most common lattice geometries are oblique, square and hexagonal. By modifying the characteristics of the S-layer through combination with functional groups and protein domains as well as their defined position and orientation to eachother (determined by the S-layer geometry) it is possible to realize various practical applications ([http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full Sleytr et al., 2007]).
Usage and Biology
S-layer proteins can be used as scaffold for nanobiotechnological applications and devices by e.g. fusing the S-layer's self-assembly domain to other functional protein domains. It is possible to coat surfaces and liposomes with S-layers. A big advantage of S-layers: after expressing in E. coli and purification, the nanobiotechnological system is cell-free. This enhances the biological security of a device.
Important parameters
| Experiment | Characteristic | Result |
|---|---|---|
| Expression (E. coli) | Localisation | Cell membrane |
| Compatibility | E. coli KRX | |
| Induction of expression | L-rhamnose for induction of T7 polymerase | |
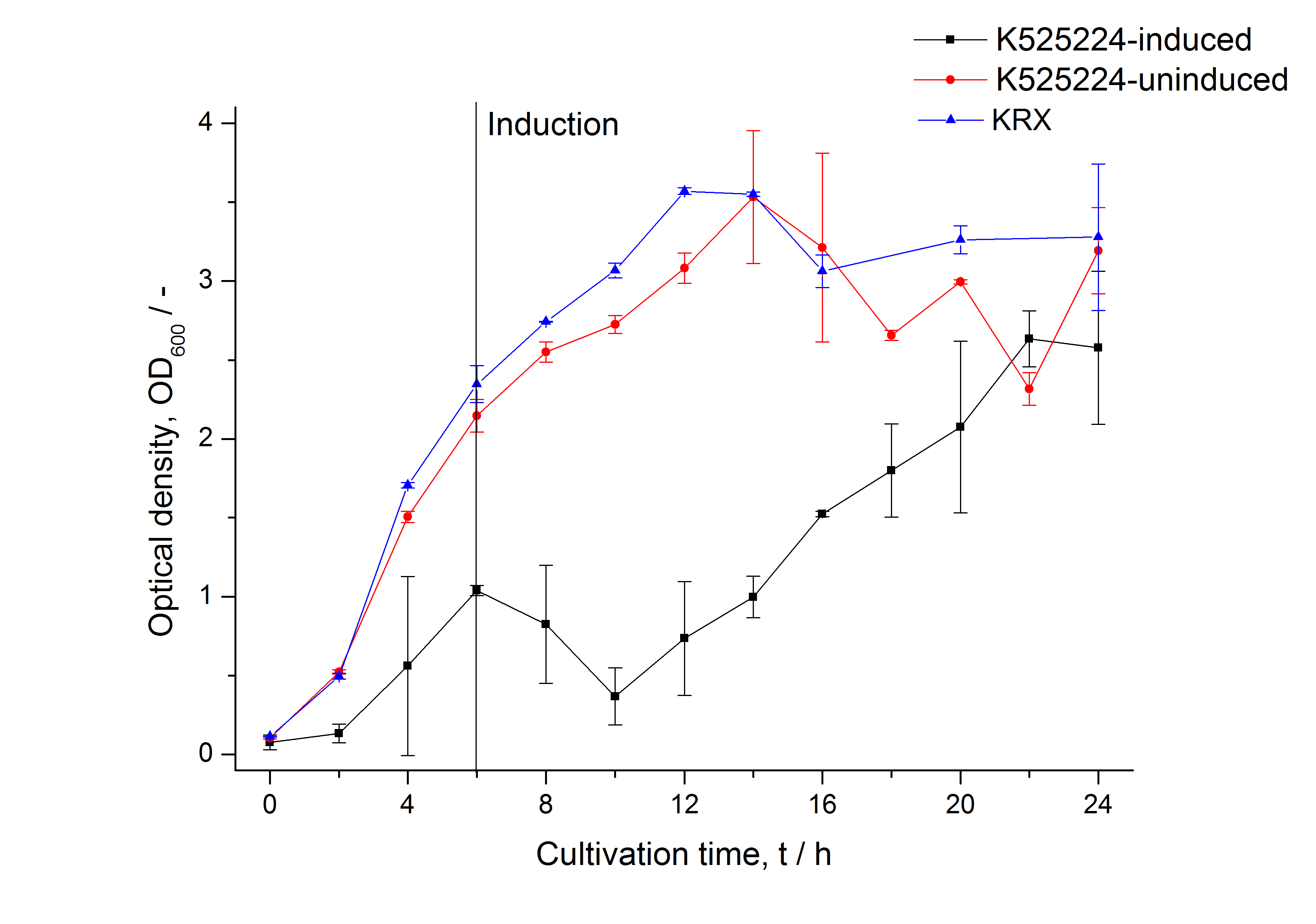
| Specific growth rate (un-/induced) | 0.251 h-1 / 0.157 h-1 | |
| Doubling time (un-/induced) | 2.76 h / 4.42 h |
After processing:
Molecular weight: 50.6 kDa
Theoretical pI: 4.25
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal PstI site found at 440
- 12INCOMPATIBLE WITH RFC[12]Illegal PstI site found at 440
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 1191
Illegal XhoI site found at 647 - 23INCOMPATIBLE WITH RFC[23]Illegal PstI site found at 440
- 25INCOMPATIBLE WITH RFC[25]Illegal PstI site found at 440
Illegal NgoMIV site found at 314
Illegal NgoMIV site found at 1403
Illegal AgeI site found at 89
Illegal AgeI site found at 305
Illegal AgeI site found at 546
Illegal AgeI site found at 593 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 995
Illegal BsaI.rc site found at 302
Illegal BsaI.rc site found at 680
Illegal BsaI.rc site found at 1082
Expression in E. coli
The CspB gen was fused with a monomeric RFP (BBa_E1010) using [http://2011.igem.org/Team:Bielefeld-Germany/Protocols#Gibson_assembly Gibson assembly] for characterization.
The CspB|mRFP fusion protein was overexpressed in E. coli KRX after induction of T7 polymerase by supplementation of 0,1 % L-rhamnose using the [http://2011.igem.org/Team:Bielefeld-Germany/Protocols/Downstream-processing#Expression_of_S-layer_genes_in_E._coli autinduction protocol] from promega.
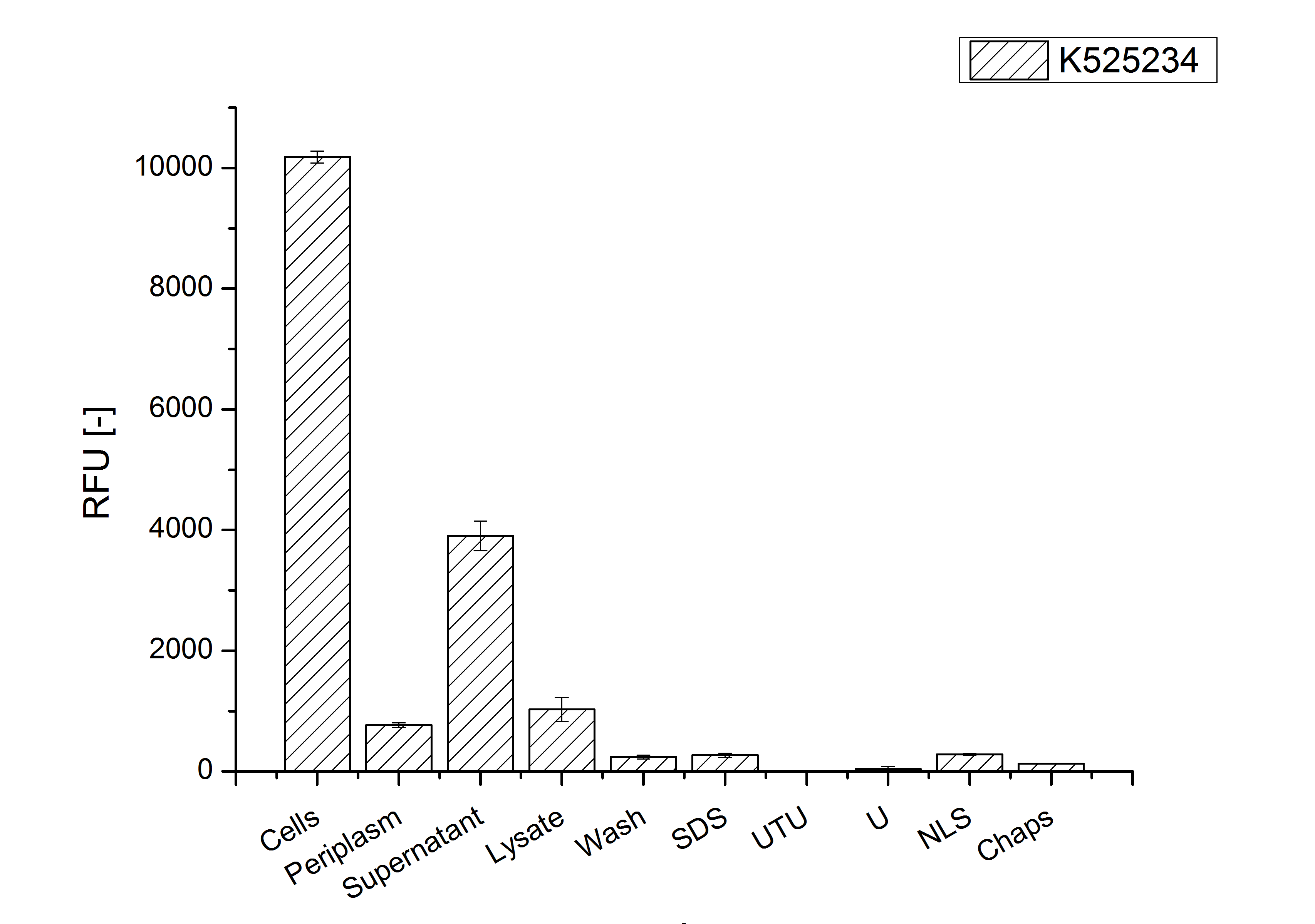
Identification and localisation
After a cultivation time of 18 h the mRFP|CspB fusion protein has to be localized in E. coli KRX. Therefor a part of the produced biomass was mechanically disrupted and the resulting lysate was wahed with ddH2O. Then the lysate was treted with ionic, nonionic and zwitterionic detergents to release the mRFP|CspB out of the membranes, if it intigrates. From the other part of the cells the periplasm was detached by using a osmotic shock.
The fluorescence in all cultivation fractions plus the fluorescence in the lysis und wash fraction shows that the fusion protein is water soluble and doesn't sediment doesn't during centrifugation. Together with the flourescence in 4 of 5 of the detergent fractions indicate that a part of the produced proteins form inclusion bodies.
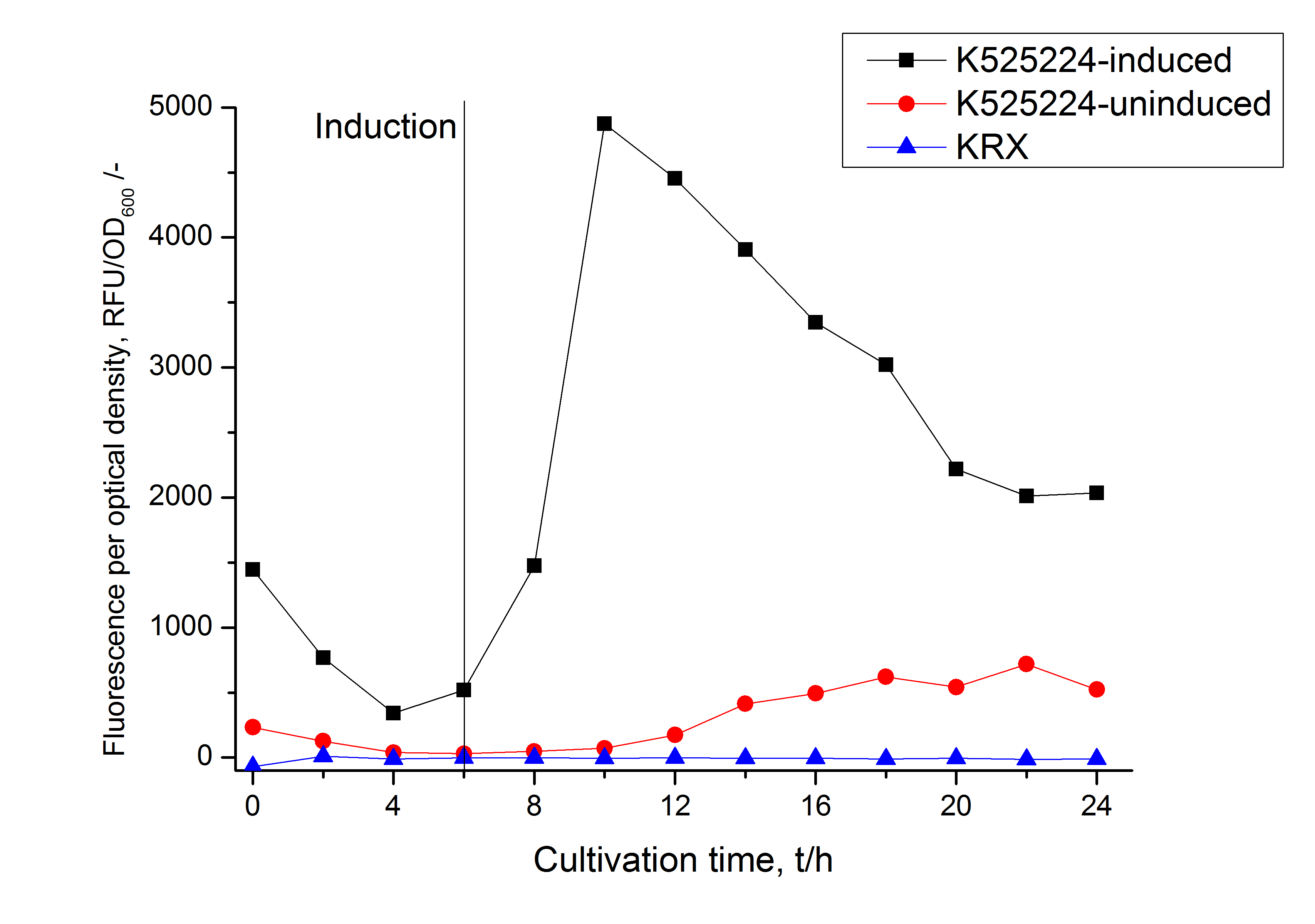
In comparison with the mRFP fusion protein of ???, wich has a lipid anchor, a minor relative fluorescence per OD600 in all cultivation and detergent fractions was detected (fig. 3). Together with the decreasing RFU/OD600 after 14 h of cultivation (fig. 2) indicates this rusult a postive effect of the lipid anchor on the protein stability.