Difference between revisions of "Part:BBa K4195034"
Siliang Zhan (Talk | contribs) (→Improved parts) |
Siliang Zhan (Talk | contribs) (→Improved parts) |
||
| (One intermediate revision by the same user not shown) | |||
| Line 6: | Line 6: | ||
===Improved by Fudan iGEM 2023 === | ===Improved by Fudan iGEM 2023 === | ||
| − | INPNC exhibits compatibility with the translocation and surface display of proteins containing multiple cofactors and disulfide bond-containing passengers. When this carrier protein comprises antigen-nanobody pairs, it can mediate specific adhesion of | + | INPNC exhibits compatibility with the translocation and surface display of proteins containing multiple cofactors and disulfide bond-containing passengers. When this carrier protein comprises antigen-nanobody pairs, it can mediate specific adhesion of ''E. coli''. Therefore, we compared the surface display efficiency of intimin and INPNC by verifying their adhesion abilities mediated by fusion with antigen and nanobody. |
====Improved parts==== | ====Improved parts==== | ||
| − | Our improved part is [https://parts.igem.org/Part:BBa_K4765108 BBa_K4765108(Twister P1 + T7_RBS + INPNC-Nb3 fusion + stem-loop)].We fused INPNC with Ag and Nb and constructed this part into our ribozyme-assisted polycistronic co-expression | + | Our improved part is [https://parts.igem.org/Part:BBa_K4765108 BBa_K4765108 (Twister P1 + T7_RBS + INPNC-Nb3 fusion + stem-loop)].We fused INPNC with Ag and Nb and constructed this part into our ribozyme-assisted polycistronic co-expression system. |
<br> | <br> | ||
Latest revision as of 10:14, 12 October 2023
INPNC-his

Improved by Fudan iGEM 2023
INPNC exhibits compatibility with the translocation and surface display of proteins containing multiple cofactors and disulfide bond-containing passengers. When this carrier protein comprises antigen-nanobody pairs, it can mediate specific adhesion of E. coli. Therefore, we compared the surface display efficiency of intimin and INPNC by verifying their adhesion abilities mediated by fusion with antigen and nanobody.
Improved parts
Our improved part is BBa_K4765108 (Twister P1 + T7_RBS + INPNC-Nb3 fusion + stem-loop).We fused INPNC with Ag and Nb and constructed this part into our ribozyme-assisted polycistronic co-expression system.
Biology
INPNC INPNC is a truncated form of ice nucleation protein (INP) consisting of N- and C-terminal domains. It is a membrane protein commonly used to display protein on the cell surface (1).
Usage and design
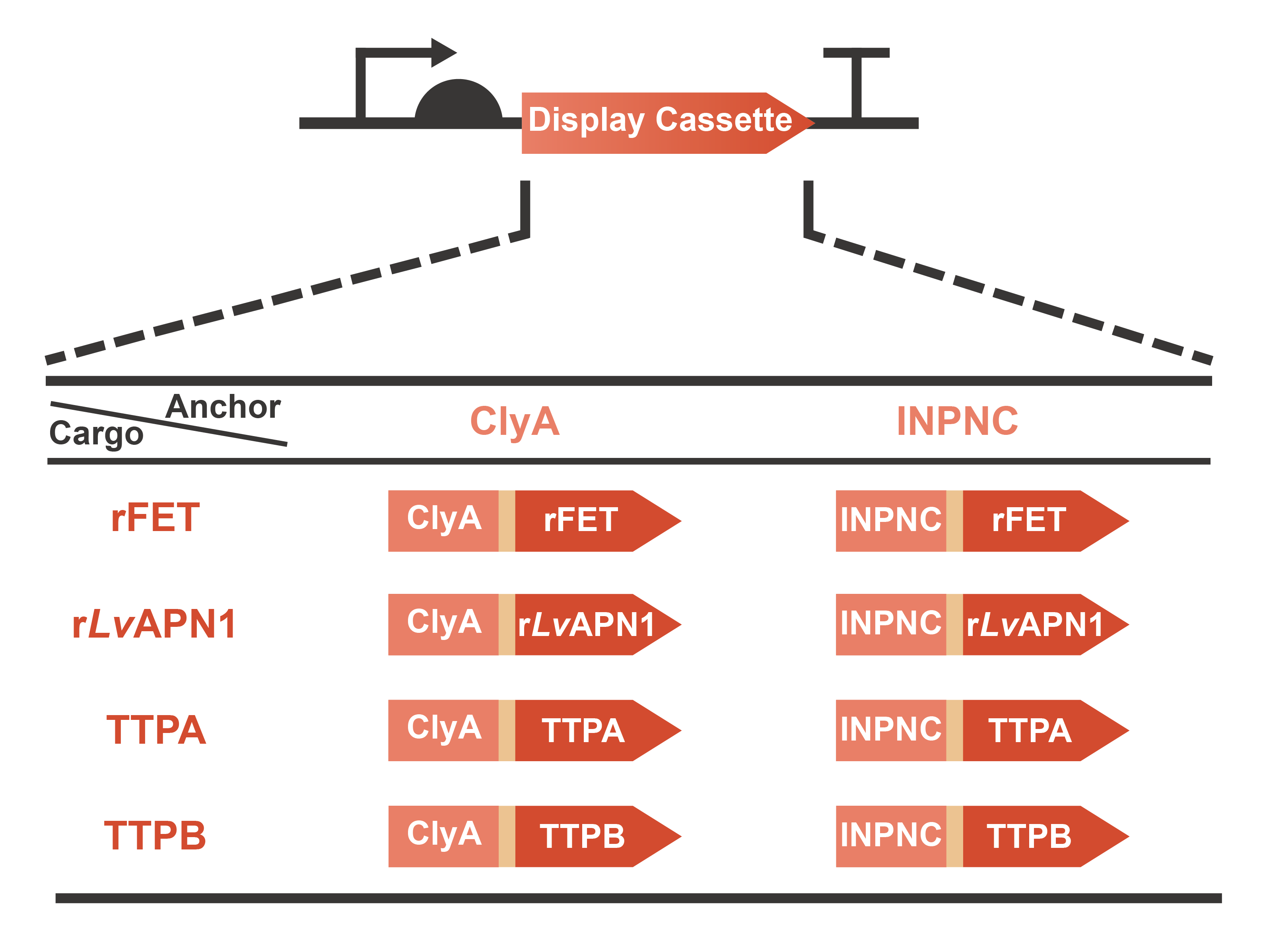
Engineering OMVs for treating and preventing AHPND caused by the pathogen V. parahaemolyticus are a significant part of OMEGA project (Operable Magic to Efficiently Getting over AHPND). Based on the efforts of our previous projects in 2020 (AnTea-Glyphosate) and 2021 (SALVAGE), we further developed the surface display system on the OMVs released by the engineered bacteria. The usage of cargo proteins was no more limited to enzymes that are usually utilized to catalyze series bio-chemical reactions, since some receptors or ligands involved in complex protein-protein interaction (PPI) were selected as the cargo candidates. This year, we chose two classic anchor proteins, ClyA and INPNC, to construct the display cassette with various cargo proteins including rFET (receptor), rLvAPN1 (receptor), TTPA (ligand) and TTPB (ligand) (Fig. 1). On one hand, with the receptors displayed, OMVs will gain the function of neutralizing toxins secreted by V. parahaemolyticus. On the other hand, with the assistance of ligands displayed on the surface, OMVs will become a special vector to deliver antimicrobials for the specific pathogen. In summary, we have taken a step closer to the collections of extracellular functional elements (EFE), combining the OMVs, secretion systems and surface display systems which we have been dedicated to since 2020. Learn more information from our Design page.
Fig. 1 Graphic description of the expression gene circuits for display cassette designed in OMEGA project.
The isolation and purification of OMVs can be achieved in many ways. Nathan et al. obtained the purified OMVs via immobilized metal affinity chromatography (IMAC) using a His-tag mutant (2). In order to purify OMVs,we added a his-tag (6×His) at the C-terminal of ClyA. We used both BBa_I0500 and BBa_B0034 to construct the expression system and obtained the composite part BBa_K4195136, which are assembled into the vector pSB1C3 by standard BioBrick assembly. The constructed plasmids were transformed into E. coli BL21(DE3), then the positive transformants were selected by chloramphenicol and confirmed by colony PCR and sequencing.
Characterization
1. Identification
After transforming the plasmid into E. coli BL21(DE3), colony PCR was used to verify that the plasmid was correct. Target bands (2680 bp) can be observed at the position between 3000 bp and 2000 bp (Fig. 2).

Fig. 2 DNA gel electrophoresis of the colony PCR products of BBa_K4195135_pSB1C3.
2. Immunofluorescence (IF)
The culture was incubated overnight. Then the FITC-labeled anti-His-Tag antibody was used to target the His-tag (6×His) displayed via ClyA, followed by measuring the fluorescence intensity (λEx= 492 nm, λEm = 528 nm) to OD600 of the culture.
The results showed that the ratio of fluorescence intensity to OD600 of positive control (bacteria harboring BBa_K4195135) is higher than that of negative control (bacteria harboring BBa_K4195119) (Fig. 3), which indicated that these two surface display systems can work well.

Fig. 3 Fluorescence intensity/OD600 of E. coli whether express the anchor protein or not (p=0.0055).
Reference
1. E. van Bloois, R. T. Winter, H. Kolmar, M. W. Fraaije, Decorating microbes: surface display of proteins on Escherichia coli. Trends Biotechnol. 29, 79-86 (2011).
2. N. J. Alves, K. B. Turner, K. A. DiVito, M. A. Daniele, S. A. Walper, Affinity purification of bacterial outer membrane vesicles (OMVs) utilizing a His-tag mutant. Res. Microbiol. 168, 139-146 (2017).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NotI site found at 481
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 330
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 72
Illegal NgoMIV site found at 405
Illegal AgeI site found at 429 - 1000COMPATIBLE WITH RFC[1000]