Difference between revisions of "Part:BBa K4214000"
| (20 intermediate revisions by one other user not shown) | |||
| Line 2: | Line 2: | ||
<partinfo>BBa_K4214000 short</partinfo> | <partinfo>BBa_K4214000 short</partinfo> | ||
| + | This part codes for human Gastric Intrinsic Factor protein (AA19-417). The gene is located in chromosome 11q12.1. This gene is a part of cobalamin transporters group. It is essential for the absorption of vitamin B12. Mutations in this gene may lead to pernicious anaemia. | ||
| − | + | In many ways, the work and IF part published in the registry by the Istanbul 2013 team (Part BBa_K1096000) served as a basis for the protein production stage of our project. We added our own IF gene (BBa_K4214000) to the registry since we built upon this part. We documented and characterised this part both on gene but also on protein levels. We removed the native signal peptide from the part since it is not required for the assays that were performed in the mammalian cells. Therefore, we built a new part BBa_K4214002. We used the BBa_K4214002 part to make a new composite part with the addition of the LacI promoter BBa_J04500 from the registry. This new part is BBa_K4214003 which is well characterised. In order to produce and purify our protein, we needed to ensure its functional expression. For that reason, we used the pelB sequence BBa_J32015 and added it to the BBa_K4214002 part creating the new composite part BBa_K4214004. | |
| − | |||
| − | [[Image:Part-BBa K4214000.png| | + | [[Image:Part-BBa K4214000.png|250px|thumb|right|Fig.: ''Human GIF (BBa_K4214000) '']] |
| − | + | ||
| + | We '''removed''' the native signal peptide (AA1-18) from the N-terminal in order to obtain a sequence that can be worked with more easily in bacterial and mammalian systems. | ||
| + | |||
| + | =Usage and Biology= | ||
| + | <hr> | ||
*NCBI page of the human gastric intrinsic factor [https://www.ncbi.nlm.nih.gov/gene/2694] | *NCBI page of the human gastric intrinsic factor [https://www.ncbi.nlm.nih.gov/gene/2694] | ||
| − | * | + | *This part was used by us to create mutants of GIF, which were used for surface display screening of autoantibody binding in our BiG-IF project |
| − | *This part of hGIF | + | *This part of hGIF does not contain the native signal peptide (AA1-18) |
| + | |||
| + | =Characterization= | ||
| + | <hr> | ||
| + | |||
| + | ===Agarose gel image of part=== | ||
| + | <div> | ||
| + | The following agarose gel image shows the results from restriction digestion of BBa_K4214000 with Bgl-II and SalI in order to continue with ligation to our expression vector. | ||
| + | |||
| + | [[Image:BBa K4214000 Gel.png|450px|thumb|left|Fig.: ''Agarose gel image showing the results from restriction digestion of BBa_K4214000 with Bgl-II and SalI in order to continue with ligation to our expression vector '']] | ||
| + | <br><br><br> | ||
| + | <br><br><br> | ||
| + | <br><br><br> | ||
| + | <br><br><br> | ||
| + | <br><br><br> | ||
| + | <br><br><br> | ||
| + | <br><br><br> | ||
| + | <br><br><br> | ||
| + | </div> | ||
| + | <hr> | ||
| + | |||
| + | ===Western blot image of part=== | ||
| + | The BBa_K4214000 part is our wild-type intrinsic factor gene and we inserted it in our expression vector. We transfected the HEK293 cells and expressed our protein in the surface of the cells. We detected our protein by using an uspecific | ||
| + | Figure A depicts the Western Blot image (fluorescent image) of our samples- Wild type plasmid, mutant 20201 plasmid and Negative control (without any plasmid) loaded at two different concentrations-15 µg and 25 µg. | ||
| + | From both Figure A and B, we observed that our protein (marked by green arrows) is being produced in mutant 20201 by the presence of light yellow bands in between 70 kDa to 55 kDa bands. The light yellow bands indicate the binding of both Myc antibody (green) and epitope unspecific IF primary antibody (red). Myc is present in the plasmid backbone and is one of the tags for confirmation for our protein. | ||
| + | |||
| + | |||
| + | [[Image:Western-blot.png|600px|thumb|center|Fig.: ''Western blot image of our samples- Wild Type, mutant 20201 and Negative control loaded with two different time points (24, 48) and concentrations (15 µg and 25 µg) '']] | ||
| + | |||
| + | <hr> | ||
| + | |||
| + | ===Fluorescence microscopy image of part=== | ||
| + | |||
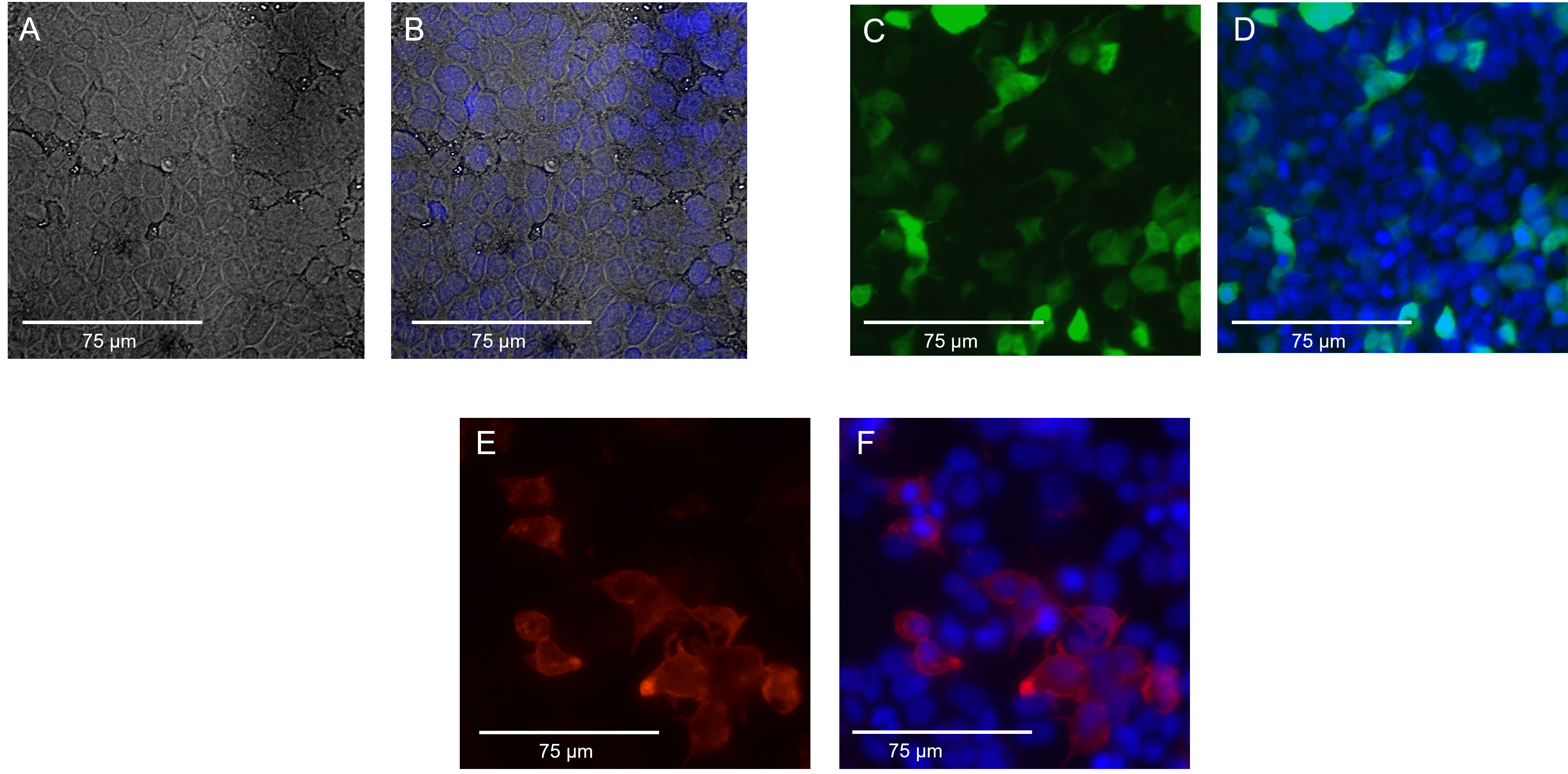
| + | From Figure A we can confirm that our cells are adherent and in good morphology. From Figure B, with the superimposition, we can establish that the blue DAPI (4′,6-diamidino-2-phenylindole) staining was successful as every cell appeared to be stained . In Figure C, we can see the bright green fluorescence courtesy of the Green Fluorescence Protein (GFP- the positive control used for transfection), confirming the success of our transfection. In Figure D, superimposing the GFP with blue DAPI was done to see the transfection efficiency visually. As one can observe, the transfection efficiency is not as high as expected for these cells. In Figure E, in the red fluorescence spectrum, we can see our IF protein. These IF proteins were tagged with primary rabbit Anti IF epitope unspecific antibody (1:100) and with secondary anti rabbit in red fluorescence (1:400). This confirms that our IF protein is expressed, thus coinciding with our western blot results as well. Figure F displays the superimposed image of DAPI staining the nuclei (blue) and the expression of IF on the surface of mutant 20201 (red). | ||
| + | |||
| + | |||
| + | [[Image:FM.png|600px|thumb|center|Fig.: ''Fluorescence microscope pictures of HEK 293T cells with part BBa_K4214000 '']] | ||
| + | |||
| + | <br><br><br> | ||
| + | |||
| + | <hr> | ||
| + | |||
| − | |||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
<partinfo>BBa_K4214000 SequenceAndFeatures</partinfo> | <partinfo>BBa_K4214000 SequenceAndFeatures</partinfo> | ||
Latest revision as of 14:55, 12 October 2022
Human Gastric Intrinsic Factor (hGIF)
This part codes for human Gastric Intrinsic Factor protein (AA19-417). The gene is located in chromosome 11q12.1. This gene is a part of cobalamin transporters group. It is essential for the absorption of vitamin B12. Mutations in this gene may lead to pernicious anaemia.
In many ways, the work and IF part published in the registry by the Istanbul 2013 team (Part BBa_K1096000) served as a basis for the protein production stage of our project. We added our own IF gene (BBa_K4214000) to the registry since we built upon this part. We documented and characterised this part both on gene but also on protein levels. We removed the native signal peptide from the part since it is not required for the assays that were performed in the mammalian cells. Therefore, we built a new part BBa_K4214002. We used the BBa_K4214002 part to make a new composite part with the addition of the LacI promoter BBa_J04500 from the registry. This new part is BBa_K4214003 which is well characterised. In order to produce and purify our protein, we needed to ensure its functional expression. For that reason, we used the pelB sequence BBa_J32015 and added it to the BBa_K4214002 part creating the new composite part BBa_K4214004.
We removed the native signal peptide (AA1-18) from the N-terminal in order to obtain a sequence that can be worked with more easily in bacterial and mammalian systems.
Usage and Biology
- NCBI page of the human gastric intrinsic factor [1]
- This part was used by us to create mutants of GIF, which were used for surface display screening of autoantibody binding in our BiG-IF project
- This part of hGIF does not contain the native signal peptide (AA1-18)
Characterization
Agarose gel image of part
The following agarose gel image shows the results from restriction digestion of BBa_K4214000 with Bgl-II and SalI in order to continue with ligation to our expression vector.
Western blot image of part
The BBa_K4214000 part is our wild-type intrinsic factor gene and we inserted it in our expression vector. We transfected the HEK293 cells and expressed our protein in the surface of the cells. We detected our protein by using an uspecific Figure A depicts the Western Blot image (fluorescent image) of our samples- Wild type plasmid, mutant 20201 plasmid and Negative control (without any plasmid) loaded at two different concentrations-15 µg and 25 µg. From both Figure A and B, we observed that our protein (marked by green arrows) is being produced in mutant 20201 by the presence of light yellow bands in between 70 kDa to 55 kDa bands. The light yellow bands indicate the binding of both Myc antibody (green) and epitope unspecific IF primary antibody (red). Myc is present in the plasmid backbone and is one of the tags for confirmation for our protein.
Fluorescence microscopy image of part
From Figure A we can confirm that our cells are adherent and in good morphology. From Figure B, with the superimposition, we can establish that the blue DAPI (4′,6-diamidino-2-phenylindole) staining was successful as every cell appeared to be stained . In Figure C, we can see the bright green fluorescence courtesy of the Green Fluorescence Protein (GFP- the positive control used for transfection), confirming the success of our transfection. In Figure D, superimposing the GFP with blue DAPI was done to see the transfection efficiency visually. As one can observe, the transfection efficiency is not as high as expected for these cells. In Figure E, in the red fluorescence spectrum, we can see our IF protein. These IF proteins were tagged with primary rabbit Anti IF epitope unspecific antibody (1:100) and with secondary anti rabbit in red fluorescence (1:400). This confirms that our IF protein is expressed, thus coinciding with our western blot results as well. Figure F displays the superimposed image of DAPI staining the nuclei (blue) and the expression of IF on the surface of mutant 20201 (red).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 265
Illegal BsaI.rc site found at 945
Illegal SapI site found at 935