Difference between revisions of "Part:BBa K3861017"
Martje1998 (Talk | contribs) |
|||
| (8 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
<partinfo>BBa_K3861017 short</partinfo> | <partinfo>BBa_K3861017 short</partinfo> | ||
| + | ==='''Introduction'''=== | ||
<html> | <html> | ||
| − | Composite part containing the PlldR promoter | + | Composite part containing the PlldR promoter (<a href="https://parts.igem.org/Part:BBa_K1847008">BBa_K1847008</a>) fused to GFP (<a href="https://parts.igem.org/Part:BBa_E0840">BBa_E0840</a>) as a reporter gene. Both parts were already characterized in depth by other teams. We used this part in <i>Salmonella</i> Typhimurium. |
</html> | </html> | ||
| Line 19: | Line 20: | ||
<partinfo>BBa_K3861017 parameters</partinfo> | <partinfo>BBa_K3861017 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
| + | |||
| + | ==='''Characterization'''=== | ||
| + | <html> | ||
| + | |||
| + | The lactate-inducible promoter (PlldR) of E. coli is frequently used and well characterized by a variety of iGEM team, highlighted by the high frequency of part-characterization on the respective part web page. Since we are working with <i>Salmonella</i> Typhimurium and wanted to use a promoter that is induced by lactate, which is secreted in high amounts by cancer cells. For our project, we therefore decided to characterize the lldR promoter (BBa_K1847008) in the S. Typhimurium background. | ||
| + | For this, we fused PlldR to the green fluorescent protein (gfp; BBa_E0840) reporter gene and cloned it into the pKF01 backbone we designed for our project. pKF01 expresses the lldR gene from a constitutive synthetic promoter (Psyn_thyA, BBa_K3861002), together with the thyA gene (BBa_K3861003). | ||
| + | We characterized the promoter by using lactic acid at different concentrations. | ||
| + | If LldR is not present, PlldR drives expression of <i>gfp</i> constitutively, which can be observed in the coloring of the colonies on LB-agar plates. | ||
| + | |||
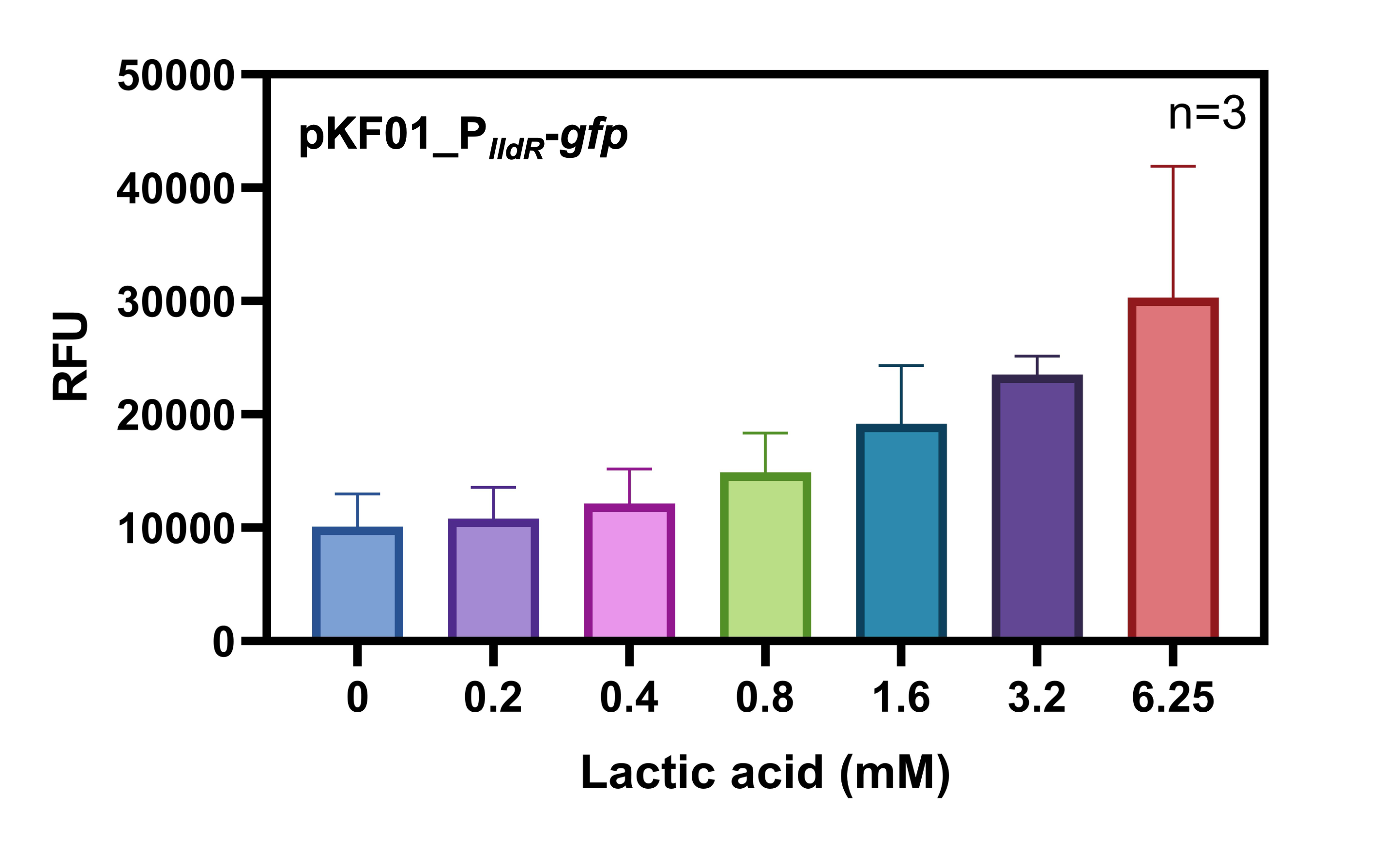
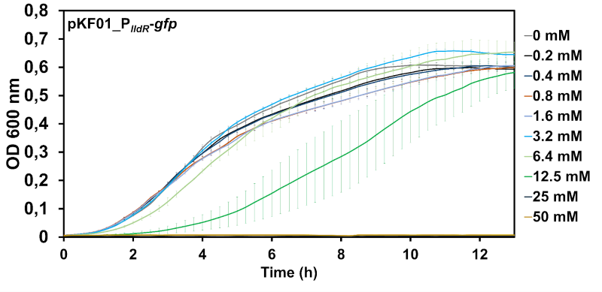
| + | When LldR, the repressor of this promoter, is present in higher amounts, the promoter becomes inducible. Here, LldR binds to the promoter, thus blocking it from being targeted by the RNA-polymerase. Upon presence of an inducer LldR releafs DNA and PlldR is free and transcription is possible. This promoter can be fine-tuned by varying different inducer concentrations (Fig. 1). The use of lactic acid for induction of the PlldR was shown to be detrimental to bacterial growth at higher concentrations, as S. Typhimurium strains were able to grow at lactic acid concentration up to 12.5 mM. At higher concentrations (25 and 50 mM lactic acid) growth was inhibited (Fig. 2). | ||
| + | |||
| + | |||
| + | <figure> | ||
| + | <img src="https://2021.igem.org/wiki/images/7/71/T--Humboldt_Berlin--dose_dependent.png" alt="PlldR-GFP" width="500"> | ||
| + | <figcaption>Fig. 1 - Characterization of the lldR promoter using GFP as reporter. RFU = relative fluorescene unit; n = number of replications.</figcaption> | ||
| + | </figure> | ||
| + | </html> | ||
| + | <html> | ||
| + | <figure> | ||
| + | <img src="https://2021.igem.org/wiki/images/4/43/T--Humboldt_Berlin--Target_Taxi_fig4.png" alt="PlldR-GFP1" width="500"> | ||
| + | <figcaption>Fig. 2 - <i>Salmonella</i> Typhimurium growth curve under different lactic acid concentrations. The optical density at a wavelength of 600 nm was measured for a period of 14 h. Growth was measured at different lactic acid concentrations (0-50 mM). </figcaption> | ||
| + | </figure> | ||
| + | </html> | ||
Latest revision as of 03:58, 22 October 2021
PlldR-GFP
Introduction
Composite part containing the PlldR promoter (BBa_K1847008) fused to GFP (BBa_E0840) as a reporter gene. Both parts were already characterized in depth by other teams. We used this part in Salmonella Typhimurium.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 78
Illegal NheI site found at 101 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 816
Characterization
The lactate-inducible promoter (PlldR) of E. coli is frequently used and well characterized by a variety of iGEM team, highlighted by the high frequency of part-characterization on the respective part web page. Since we are working with Salmonella Typhimurium and wanted to use a promoter that is induced by lactate, which is secreted in high amounts by cancer cells. For our project, we therefore decided to characterize the lldR promoter (BBa_K1847008) in the S. Typhimurium background.
For this, we fused PlldR to the green fluorescent protein (gfp; BBa_E0840) reporter gene and cloned it into the pKF01 backbone we designed for our project. pKF01 expresses the lldR gene from a constitutive synthetic promoter (Psyn_thyA, BBa_K3861002), together with the thyA gene (BBa_K3861003).
We characterized the promoter by using lactic acid at different concentrations.
If LldR is not present, PlldR drives expression of gfp constitutively, which can be observed in the coloring of the colonies on LB-agar plates.
When LldR, the repressor of this promoter, is present in higher amounts, the promoter becomes inducible. Here, LldR binds to the promoter, thus blocking it from being targeted by the RNA-polymerase. Upon presence of an inducer LldR releafs DNA and PlldR is free and transcription is possible. This promoter can be fine-tuned by varying different inducer concentrations (Fig. 1). The use of lactic acid for induction of the PlldR was shown to be detrimental to bacterial growth at higher concentrations, as S. Typhimurium strains were able to grow at lactic acid concentration up to 12.5 mM. At higher concentrations (25 and 50 mM lactic acid) growth was inhibited (Fig. 2).