Difference between revisions of "Part:BBa K3893030"
JeanHerdoiza (Talk | contribs) |
JeanHerdoiza (Talk | contribs) (Undo revision 581166 by JeanHerdoiza (talk)) |
||
| (25 intermediate revisions by the same user not shown) | |||
| Line 18: | Line 18: | ||
| − | |||
===Usage and Biology=== | ===Usage and Biology=== | ||
| + | This device is a population oscillator based on quorum sensing and a lysis protein coded by gene φX174E. Quorum sensing is a cell-to-cell communication mechanism via 3OC6 homo-serine lactone (AHL) molecules that passively diffuse across the cell membrane. | ||
| + | For a small number of cells in the population, the level of AHL produced is low and the transcription factor LuxR-AHL does not activate the production of the lysis protein. When population size increases, the level of AHL in the culture media increases reaching a threshold and activating the production of the lysis protein. In turn, when there is enough amount of lysis protein, the cells start to lyse and die, reducing the size of the population. Under the threshold of the pLux promoter, the production of the lysis protein gets de-activated and cells start to grow again. | ||
| + | |||
| + | |||
| + | == Part engineering and DBTL cycle == | ||
| + | <html> | ||
| + | The development of this part was performed following the steps of the <a href="https://2021.igem.org/Team:Ecuador/Engineering">Design-Build-Test-Learn (DBTL) cycle of synthetic biology</a>. Here, we describe the different steps and the results obtained. | ||
| + | </html> | ||
| + | === Design === | ||
| + | <html> | ||
| + | This part is designed for delivering and releasing dsRNA molecules of the gene of interest in the context of controlled oscillations in the size of the bacterial population. However, we replaced the gene of interest with a reporter to test the effectiveness of part and to use it as a proxy to estimate the amount of lysis protein being expressed. To this end, LuxR-AHL transcription factor activates simultaneously the expression of GFP and the lysis protein PhiX174E. | ||
| + | <div style="float:center"> | ||
| + | <img style="width: 60%" src="https://2021.igem.org/wiki/images/1/18/T--Ecuador--Eng_DBcircuit.png"> | ||
| + | </div> | ||
| + | |||
| + | <p> | ||
| + | Before building our device in the lab, we made a <a href='https://2021.igem.org/Team:Ecuador/Model?ref-tab=tab2#m2model'>mathematical ODE model</a> to check this design was able to produce the desired oscillations. Although the ODE model only considers N=20 cells, we see qualitatively confirm that our device is able to develop the desired behaviour, i.e. the population oscillations based on quorum sensing.</p> | ||
| + | |||
| + | <b>ODE Model</b> | ||
| + | <p>As the ODE model of Module 2-Delivery and Release, this new model has 7 states. Particularly N is the number cells growing in the culture, and GFP is the system output. Ae is the number of molecules in the culture medium, and the remaining species are intracellular ones. | ||
| + | </p> | ||
| + | <div style="float:center"> | ||
| + | <img style="width: 60%" src="https://2021.igem.org/wiki/images/e/e8/T--Ecuador--EngModel.png"> </div> | ||
| + | <br /> | ||
| + | <b>Computational simulation</b><p>From the computational simulation performed with our model (see the details in our <a href='https://2021.igem.org/Team:Ecuador/Model?ref-tab=tab2#m2model'>modeling page</a>), as expected, we can confirm that the designed gene circuit shows an oscillatory behaviour since cells die only when the lysis protein is activated. Therefore, this ODE model used as a proxy of Module 2-Delivery and Release fulfills the requirements for delivering and releasing dsRNA molecules due to cell lysis. | ||
| + | </p> | ||
| + | <div style="float:center"> | ||
| + | <img style="width: 60%" src="https://2021.igem.org/wiki/images/d/da/T--Ecuador--EngOscilatorGFP.png"></div> | ||
| + | |||
| + | </html> | ||
| + | |||
| + | === Build === | ||
| + | <html><p> | ||
| + | In the lab, we needed to build our device. | ||
| + | For this we planned a golden gate assembly with 3 levels using the <a href="http://2018.igem.org/Team:Valencia_UPV/Design#GB">Golden Braid assembly method </a> from Valencia_UPV. First, we combined together two composite parts that were already in the Part Registry (<a href="https://parts.igem.org/Part:BBa_K2656122">BBa_K2656122 </a> a Level 1 transcriptional unit expresing GFP under the control of the pux promoter and <a href="https://parts.igem.org/Part:BBa_K2656114">BBa_K2656114 </a> a Level 1 transcriptional unit constitutively expressing luxR gene) to create part <a href="https://parts.igem.org/Part:BBa_K3893028">BBa_K3893028 </a>. | ||
| + | </p> | ||
| + | <div style="float:center"> | ||
| + | <a href="https://parts.igem.org/Part:BBa_K3893028"> | ||
| + | <img style="width: 60%" src="https://static.igem.org/mediawiki/parts/d/d7/T--Ecuador--K3893028_sbolv.png"></a> | ||
| + | </div> | ||
| + | <p> | ||
| + | Then we created 2 more Level 1 Transcripcional units using basic parts from the <a href="http://2018.igem.org/Team:Valencia_UPV/Parts">Valencia_UPV Part Collection</a>: parts <a href="https://parts.igem.org/Part:BBa_K3893026">BBa_K3893026 </a> (a Level 1 transcriptional unit constitutively expressing luxI gene) and <a href="https://parts.igem.org/Part:BBa_K3893027">BBa_K3893027 </a> (a Level 1 transcriptional unit expresing phiX174 lysis protein under the control of the pLux promoter). This way, we combined these two parts and we created a new composite part <a href="https://parts.igem.org/Part:BBa_K3893029">BBa_K3893029</a>. | ||
| + | <div style="float:center"> | ||
| + | <a href="https://parts.igem.org/Part:BBa_K3893029"> | ||
| + | <img style="width: 60%" src="https://static.igem.org/mediawiki/parts/8/8d/T--Ecuador--K3893029_sbolv.png"></a> | ||
| + | </div> | ||
| + | |||
| + | Finally, combining these two Level 2 parts we obtained a construction with four transcriptional units implementing our initial design <a href="https://parts.igem.org/Part:BBa_K3893030">BBa_K3893030</a>. | ||
| + | |||
| + | </html> | ||
| + | |||
| + | === Test === | ||
| + | <html> | ||
| + | <p> | ||
| + | We designed a temporal experiment to assess and quantify (if possible) cell lysate. We collected absorbance and fluorescence data from the gene circuit in vivo. One of the assays is shown in the Figure below. <br /><b>Figures A and B</b> depict how cells death when the lysis protein is activated by the lux promoter K2656003. The number of cells was calibrated using standardized particle units from the Engineering Committee (Measurement Committee) and the iGEM Interlab study 2018-2019. </p> | ||
| + | <p> | ||
| + | The total GFP fluorescence expressed by the population and a single-cell is shown in <b>Figures C and D</b>, respectively. At the beginning of the experiment, the number of cells producing GFP is very low (OD 600=3.05e-6). But after lysis, GFP molecules are released to the medium and some cells are still producing GFP. We used MEFL/Particle (molecules of equivalent fluorescein per particle) as a standardized unit to quantify GFP expression per cell.</p> | ||
| + | |||
| + | <div style="float:center"> | ||
| + | <img style="width: 60%" src="https://2021.igem.org/wiki/images/thumb/1/1f/T--Ecuador--EngOscilator2.png/609px-T--Ecuador--EngOscilator2.png"></div> | ||
| + | |||
| + | <b>Materials and Methods</b> | ||
| + | <font size="-1"> | ||
| + | <p> | ||
| + | <ol> | ||
| + | <li>All experimental measurements were taken with Biotek CytationTM 3, using a 96-well plate. The experimental conditions used in this work are in the Table below</li> | ||
| + | <li>The cells were E. cloni® cells (Lucigen) transformed with the corresponding plasmid.</li> | ||
| + | <li>For all experiments, we worked with an isolated colony that contains the corresponding plasmid.</li> | ||
| + | <li>Cultures of 3mL falcon tubes with sLB medium with the corresponding antibiotic were prepared and incubated overnight (18 hours) at 37°C, 200 rpm.</li> | ||
| + | <li>The overnight cultures were refreshed in 3 mL of sBL with the corresponding antibiotic and incubated at 37°C, 200 rpm for 4 hours. </li> | ||
| + | <li>Culture tubes at OD600=0.05 were sit in cold water for 30 mins.</li> | ||
| + | <li>We measured absorbance and fluorescence of four replicas of the sample starting with OD600=3.05e-6 during 14 hours.</li> | ||
| + | <li>PROTOCOL: Time between measures 5 min, Temperature 37°C, Shaking Double orbital (Continuously), Absorbance wavelength 600 nm, Excitation wavelength 485 nm, Emission wavelength 528 nm, 96-well volume (individual) 200 μl. | ||
| + | </ol> | ||
| + | </p> | ||
| + | </font> | ||
| + | |||
| + | |||
| + | </html> | ||
| + | |||
| + | === Learn === | ||
| + | <html> | ||
| + | <p> | ||
| + | In parallel to this DBTL cycle to develop our Module 2, we performed a smaller inned DBTL cycle including design, assembly (build), measurements (test), and model and characterization of the part (learn) using part <a href="https://parts.igem.org/Part:BBa_K3893028">BBa_K3893028</a> and <a href="https://2021.igem.org/Team:Ecuador/Contribution">contributing to characterize the pLux promoter</a> (Part <a href="https://parts.igem.org/Part:BBa_K2656003">BBa_K2656003</a> which is the Golden Braid compatible, together with its Biobrick starndard sister part <a href="https://parts.igem.org/Part:BBa_R0062">BBa_R0062</a>) in the context of the transcriptional unit built in part <a href="https://parts.igem.org/Part:BBa_K2656122">BBa_K2656122</a>. | ||
| + | </p> | ||
| + | |||
| + | <div style="float:center"><a href="https://2021.igem.org/Team:Ecuador/Contribution"> | ||
| + | <img style="width: 60%" src="https://static.igem.org/mediawiki/parts/thumb/d/dc/T--Ecuador_UPV--Model_pLux_Charact.png/320px-T--Ecuador_UPV--Model_pLux_Charact.png"></a></div> | ||
| + | |||
| + | <p>Using the information we obtained from this smaller DBTL, we could characterize the pLux promoter (in the context of the transcriptional units data was taken, which is the same as the context of our oscillator). From this characterization of the pLux promoter in the mentioned context, we extracted the following parameters and used them to learn:</p> | ||
| + | |||
| + | <font size="-1"> | ||
| + | <p> | ||
| + | |||
| + | <ol> | ||
| + | <li>Effective dissociation constant: Kd = 15.34 nM </li> | ||
| + | <li> Hill coefficient: n = 0.958</li> | ||
| + | <li> Basal expression: 𝛽 = 0.1049</li> | ||
| + | </ol> | ||
| + | |||
| + | </p></font> | ||
| + | <p>With these parameters from the experimental data and model of pLux promoter, we went back to our model and learned, by including the new values and performing new computational simulations. </p> | ||
| + | |||
| + | <div style="float:center"> | ||
| + | <img style="width: 60%"src="https://2021.igem.org/wiki/images/2/29/T--Ecuador--M2simulation_with_param.png"> | ||
| + | </div> | ||
| + | |||
| + | <p> Here, we show the results of the new computational simulations. And we can see that the information we learned and plugged back into the initial model makes our model better. By comparing the <b>in silico vs. in vivo results</b>, we see that our model captures the temporal dynamics of the oscillator, and also recapitulates with the Total dsRNA being similar to the Total GFP in the experimental data. </p> | ||
| + | |||
| + | <div> | ||
| + | <a href="https://static.igem.org/mediawiki/2021/9/9e/T--Ecuador--Model_Exp_Comparison.png" target="_blank"> | ||
| + | <img src="https://2021.igem.org/wiki/images/thumb/9/9e/T--Ecuador--Model_Exp_Comparison.png/800px-T--Ecuador--Model_Exp_Comparison.png"> </a> | ||
| + | </div> | ||
| + | |||
| + | <p> An interesting point is that we did not fit the oscillator model with data from the experiment from part <a href="https://parts.igem.org/Part:BBa_K3893030">BBa_K3893030</a>, but we incorporated the characterization made of one of the components of the system, and still, we got good agreement between the model of Module 2 and its corresponding experimental data. This, speaks itself about the power of modeling and the DBTL cycle in Synthetic Biology. | ||
| + | Finally, our newly adjusted ODE model can be used to redesign our device, by predicting outcomes for different conditions of components, and also it can be used in an optimization process to improve other aspects of our devices we need to improve. Now we have a starting point for optimization that can be done in silico, instead of building a new set of circuits to see which one performs better than the original. </p> | ||
| + | |||
| + | </html> | ||
<!-- --> | <!-- --> | ||
| + | |||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
<partinfo>BBa_K3893030 SequenceAndFeatures</partinfo> | <partinfo>BBa_K3893030 SequenceAndFeatures</partinfo> | ||
Latest revision as of 02:19, 22 October 2021
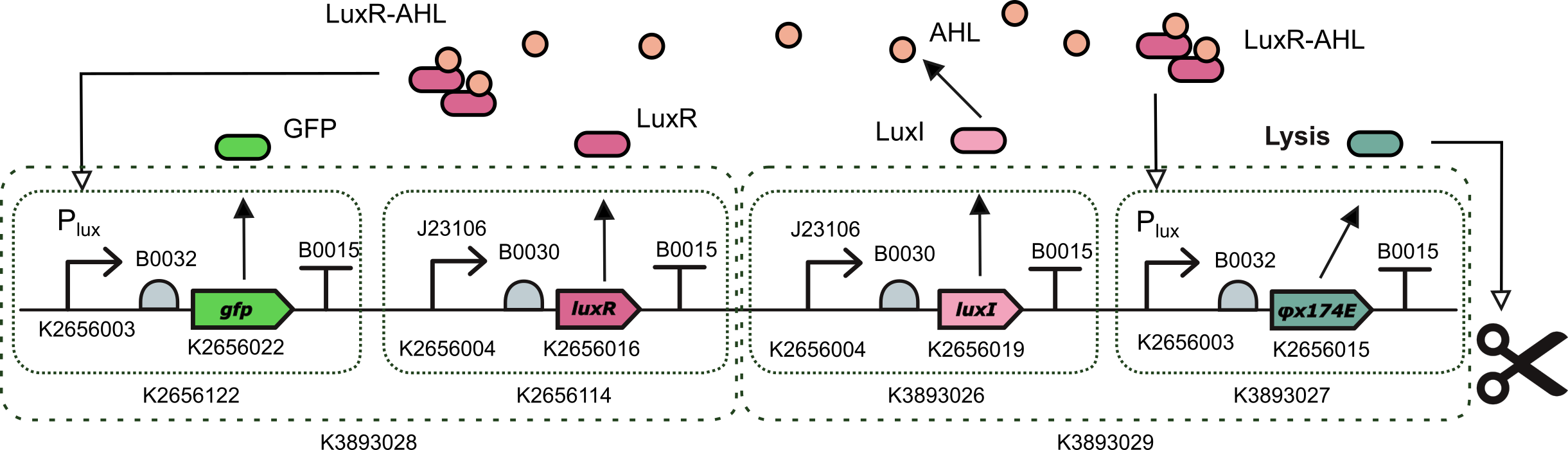
Population control device (QS-based lysis protein oscilator)
This device is an AHL induced transcriptional unit to express phiX174 lysis protein together with a TU for the constitutive expression of LuxI. It was assembled with a one-pot Level 2 Golden Gate reaction using BsmBI type IIS endonuclease.
This device is composed of the following standardized composite parts:
- BBa_K3893028: a Level 2 transcriptional unit constitutively expressing luxI gene (Golden Braid compatible)
- BBa_K3893029: a Level 2 transcriptional unit expresing phiX174 lysis protein under the control of the pux promoter (Golden Braid compatible)
Usage and Biology
This device is a population oscillator based on quorum sensing and a lysis protein coded by gene φX174E. Quorum sensing is a cell-to-cell communication mechanism via 3OC6 homo-serine lactone (AHL) molecules that passively diffuse across the cell membrane. For a small number of cells in the population, the level of AHL produced is low and the transcription factor LuxR-AHL does not activate the production of the lysis protein. When population size increases, the level of AHL in the culture media increases reaching a threshold and activating the production of the lysis protein. In turn, when there is enough amount of lysis protein, the cells start to lyse and die, reducing the size of the population. Under the threshold of the pLux promoter, the production of the lysis protein gets de-activated and cells start to grow again.
Part engineering and DBTL cycle
The development of this part was performed following the steps of the Design-Build-Test-Learn (DBTL) cycle of synthetic biology. Here, we describe the different steps and the results obtained.
Design
This part is designed for delivering and releasing dsRNA molecules of the gene of interest in the context of controlled oscillations in the size of the bacterial population. However, we replaced the gene of interest with a reporter to test the effectiveness of part and to use it as a proxy to estimate the amount of lysis protein being expressed. To this end, LuxR-AHL transcription factor activates simultaneously the expression of GFP and the lysis protein PhiX174E.

Before building our device in the lab, we made a mathematical ODE model to check this design was able to produce the desired oscillations. Although the ODE model only considers N=20 cells, we see qualitatively confirm that our device is able to develop the desired behaviour, i.e. the population oscillations based on quorum sensing.
ODE ModelAs the ODE model of Module 2-Delivery and Release, this new model has 7 states. Particularly N is the number cells growing in the culture, and GFP is the system output. Ae is the number of molecules in the culture medium, and the remaining species are intracellular ones.

Computational simulation
From the computational simulation performed with our model (see the details in our modeling page), as expected, we can confirm that the designed gene circuit shows an oscillatory behaviour since cells die only when the lysis protein is activated. Therefore, this ODE model used as a proxy of Module 2-Delivery and Release fulfills the requirements for delivering and releasing dsRNA molecules due to cell lysis.

Build
In the lab, we needed to build our device. For this we planned a golden gate assembly with 3 levels using the Golden Braid assembly method from Valencia_UPV. First, we combined together two composite parts that were already in the Part Registry (BBa_K2656122 a Level 1 transcriptional unit expresing GFP under the control of the pux promoter and BBa_K2656114 a Level 1 transcriptional unit constitutively expressing luxR gene) to create part BBa_K3893028 .
Then we created 2 more Level 1 Transcripcional units using basic parts from the Valencia_UPV Part Collection: parts BBa_K3893026 (a Level 1 transcriptional unit constitutively expressing luxI gene) and BBa_K3893027 (a Level 1 transcriptional unit expresing phiX174 lysis protein under the control of the pLux promoter). This way, we combined these two parts and we created a new composite part BBa_K3893029.
Finally, combining these two Level 2 parts we obtained a construction with four transcriptional units implementing our initial design BBa_K3893030.Test
We designed a temporal experiment to assess and quantify (if possible) cell lysate. We collected absorbance and fluorescence data from the gene circuit in vivo. One of the assays is shown in the Figure below.
Figures A and B depict how cells death when the lysis protein is activated by the lux promoter K2656003. The number of cells was calibrated using standardized particle units from the Engineering Committee (Measurement Committee) and the iGEM Interlab study 2018-2019.
The total GFP fluorescence expressed by the population and a single-cell is shown in Figures C and D, respectively. At the beginning of the experiment, the number of cells producing GFP is very low (OD 600=3.05e-6). But after lysis, GFP molecules are released to the medium and some cells are still producing GFP. We used MEFL/Particle (molecules of equivalent fluorescein per particle) as a standardized unit to quantify GFP expression per cell.

- All experimental measurements were taken with Biotek CytationTM 3, using a 96-well plate. The experimental conditions used in this work are in the Table below
- The cells were E. cloni® cells (Lucigen) transformed with the corresponding plasmid.
- For all experiments, we worked with an isolated colony that contains the corresponding plasmid.
- Cultures of 3mL falcon tubes with sLB medium with the corresponding antibiotic were prepared and incubated overnight (18 hours) at 37°C, 200 rpm.
- The overnight cultures were refreshed in 3 mL of sBL with the corresponding antibiotic and incubated at 37°C, 200 rpm for 4 hours.
- Culture tubes at OD600=0.05 were sit in cold water for 30 mins.
- We measured absorbance and fluorescence of four replicas of the sample starting with OD600=3.05e-6 during 14 hours.
- PROTOCOL: Time between measures 5 min, Temperature 37°C, Shaking Double orbital (Continuously), Absorbance wavelength 600 nm, Excitation wavelength 485 nm, Emission wavelength 528 nm, 96-well volume (individual) 200 μl.
Learn
In parallel to this DBTL cycle to develop our Module 2, we performed a smaller inned DBTL cycle including design, assembly (build), measurements (test), and model and characterization of the part (learn) using part BBa_K3893028 and contributing to characterize the pLux promoter (Part BBa_K2656003 which is the Golden Braid compatible, together with its Biobrick starndard sister part BBa_R0062) in the context of the transcriptional unit built in part BBa_K2656122.
Using the information we obtained from this smaller DBTL, we could characterize the pLux promoter (in the context of the transcriptional units data was taken, which is the same as the context of our oscillator). From this characterization of the pLux promoter in the mentioned context, we extracted the following parameters and used them to learn:
- Effective dissociation constant: Kd = 15.34 nM
- Hill coefficient: n = 0.958
- Basal expression: 𝛽 = 0.1049
With these parameters from the experimental data and model of pLux promoter, we went back to our model and learned, by including the new values and performing new computational simulations.

Here, we show the results of the new computational simulations. And we can see that the information we learned and plugged back into the initial model makes our model better. By comparing the in silico vs. in vivo results, we see that our model captures the temporal dynamics of the oscillator, and also recapitulates with the Total dsRNA being similar to the Total GFP in the experimental data.
An interesting point is that we did not fit the oscillator model with data from the experiment from part BBa_K3893030, but we incorporated the characterization made of one of the components of the system, and still, we got good agreement between the model of Module 2 and its corresponding experimental data. This, speaks itself about the power of modeling and the DBTL cycle in Synthetic Biology. Finally, our newly adjusted ODE model can be used to redesign our device, by predicting outcomes for different conditions of components, and also it can be used in an optimization process to improve other aspects of our devices we need to improve. Now we have a starting point for optimization that can be done in silico, instead of building a new set of circuits to see which one performs better than the original.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 956
Illegal NheI site found at 979
Illegal NheI site found at 1914
Illegal NheI site found at 1937 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]