Difference between revisions of "Part:BBa K3598000"
| (4 intermediate revisions by 2 users not shown) | |||
| Line 5: | Line 5: | ||
AOX1 is a very common inducible promoter in yeast, and its ON/OFF condition is controlled by methanol. | AOX1 is a very common inducible promoter in yeast, and its ON/OFF condition is controlled by methanol. | ||
| − | |||
| − | |||
| − | |||
===Performance of AOX1 in in Pichia pastoris === | ===Performance of AOX1 in in Pichia pastoris === | ||
| − | |||
| − | In our project, we | + | In our project, we choose AOX1 promoter (BBa_I764001:[[Part:BBa_I764001|AOX1 Promoter]]), which is a common inducible promoter used in Pichia pastoris GS115, to control the expression. To make sure the AOX1 promoter works properly, we tested its performance with sfGFP. |
| − | We | + | We added sfGFP gene behind AOX1 promoter, inserted the whole sequence into pPIC9K backbone and transformed the plasmid into P. pastoris GS115. The recombinant strain was conducted fermentation test BMMY Medium. We measured the OD600 absorbance and fluorescence of the fermentation broth every 24 hours, and the supernatant samples during the fermentation were verified through SDS-PAGE gel electrophoresis. |
<b>Growth curve and fluorescence test</b> | <b>Growth curve and fluorescence test</b> | ||
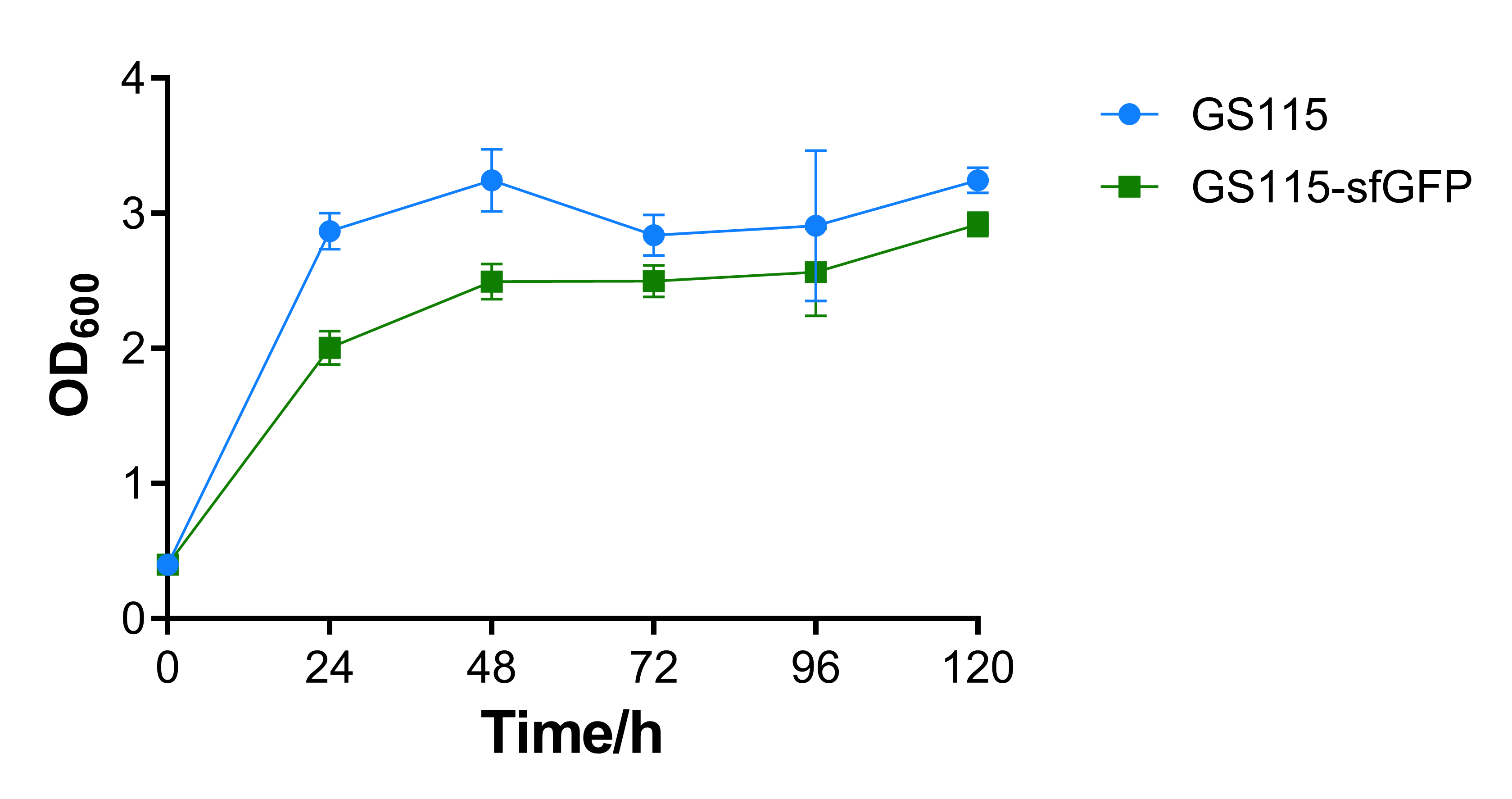
| − | The OD600 of the recombinant strain | + | The OD600 absorbance of the recombinant strain that contains sfGFP is a little lower than the control strain P. pastoris GS115. That may be caused by the additional expression of sfGFP, the results show that expression of heterologous gene would repress cell growth, while the repression is not intensive. |
| − | [[File:T-- | + | [[File:T--BEIJING_4ELEVEN--Contribution_Figure_1_OD600.png|600px|thumb|center|Figure 1. OD600 absorbance of P. pastoris GS115 and recombinant strain that contains sfGFP every 24 hours.]] |
| − | + | ||
| − | + | ||
| − | + | ||
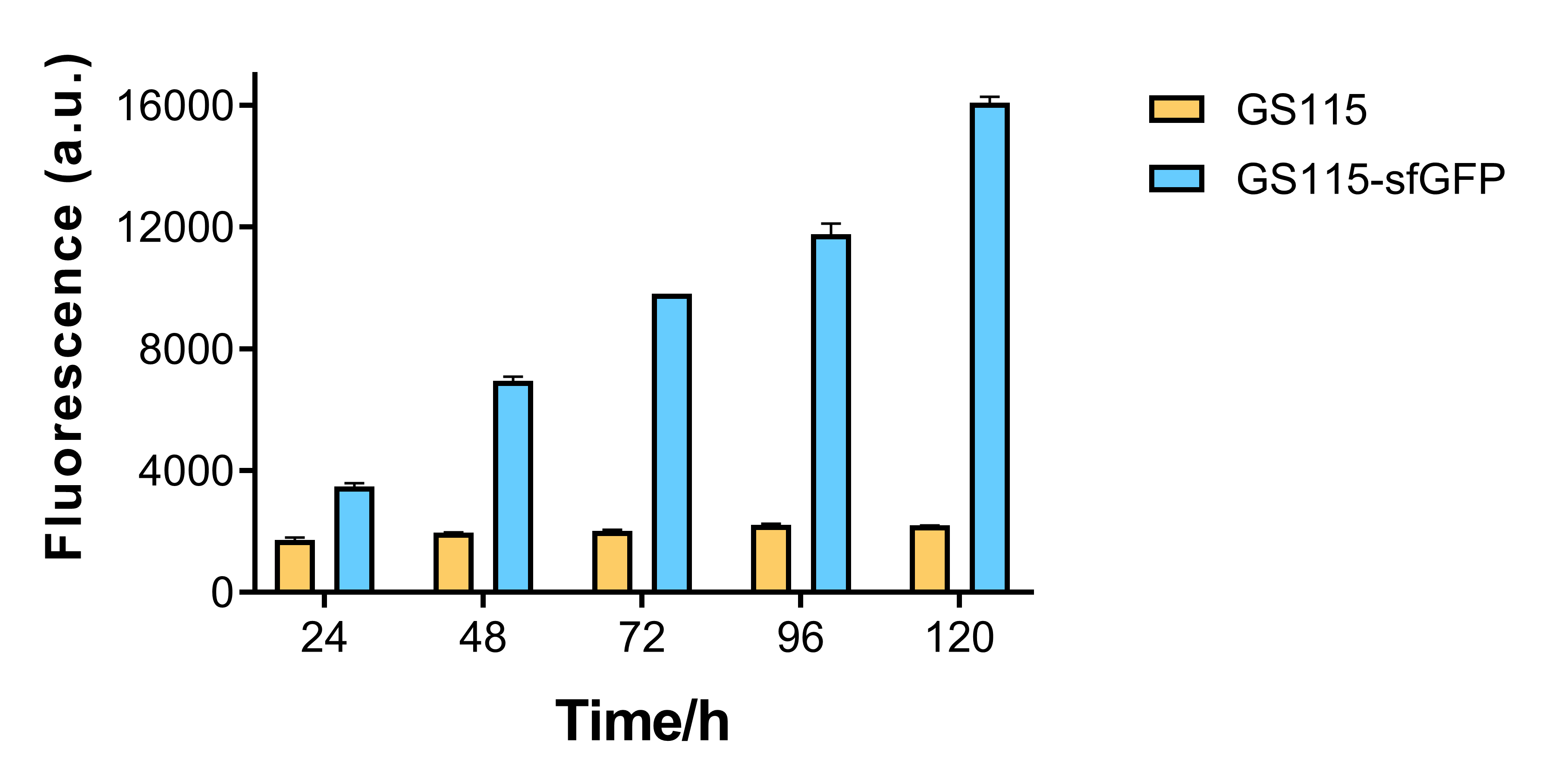
The fluorescence of recombinant strain that contains sfGFP becomes higher for every 24 hours, while that of P. pastoris GS115 remains mostly the same. This shows that AOX1 promoter under the control of methanol has the capability of promoting fluorescent protein expression. | The fluorescence of recombinant strain that contains sfGFP becomes higher for every 24 hours, while that of P. pastoris GS115 remains mostly the same. This shows that AOX1 promoter under the control of methanol has the capability of promoting fluorescent protein expression. | ||
| − | + | [[File:T--BEIJING 4ELEVEN--Contribution2.png|600px|thumb|center|Figure 2. Fluorescence of P. pastoris GS115 and recombinant strain that contains sfGFP every 24 hours.]] | |
<b> SDS-PAGE gel analysis </b> | <b> SDS-PAGE gel analysis </b> | ||
| − | |||
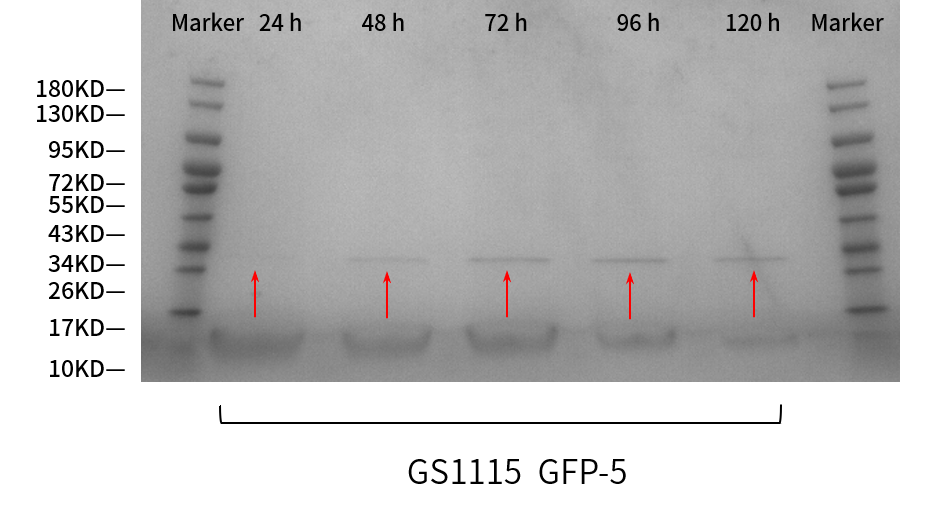
| + | From the results (Figure 3), correct bands of sfGFP were shown as expected. The protein band at 24h can be barely seen; that at 48h is greater; and at 72, 96, 120h are nearly the same, all greater than at 48 h. So the clarity of the bands increases over time, which means the protein expression is getting more intensive every 24 hours. | ||
| + | |||
| + | |||
| + | [[File:T--BEIJING 4ELEVEN--Contribution3.png|600px|thumb|center|Figure 3. SDS-PAGE gel analysis of supernatant samples during the fermentation]] | ||
| + | |||
| + | <b> Conclusion </b> | ||
| + | |||
| + | All the results prove that AOX1 promoter works properly. | ||
| − | |||
Latest revision as of 08:08, 27 October 2020
AOX1 promoter
AOX1 is a very common inducible promoter in yeast, and its ON/OFF condition is controlled by methanol.
Performance of AOX1 in in Pichia pastoris
In our project, we choose AOX1 promoter (BBa_I764001:AOX1 Promoter), which is a common inducible promoter used in Pichia pastoris GS115, to control the expression. To make sure the AOX1 promoter works properly, we tested its performance with sfGFP.
We added sfGFP gene behind AOX1 promoter, inserted the whole sequence into pPIC9K backbone and transformed the plasmid into P. pastoris GS115. The recombinant strain was conducted fermentation test BMMY Medium. We measured the OD600 absorbance and fluorescence of the fermentation broth every 24 hours, and the supernatant samples during the fermentation were verified through SDS-PAGE gel electrophoresis.
Growth curve and fluorescence test
The OD600 absorbance of the recombinant strain that contains sfGFP is a little lower than the control strain P. pastoris GS115. That may be caused by the additional expression of sfGFP, the results show that expression of heterologous gene would repress cell growth, while the repression is not intensive.
The fluorescence of recombinant strain that contains sfGFP becomes higher for every 24 hours, while that of P. pastoris GS115 remains mostly the same. This shows that AOX1 promoter under the control of methanol has the capability of promoting fluorescent protein expression.
SDS-PAGE gel analysis
From the results (Figure 3), correct bands of sfGFP were shown as expected. The protein band at 24h can be barely seen; that at 48h is greater; and at 72, 96, 120h are nearly the same, all greater than at 48 h. So the clarity of the bands increases over time, which means the protein expression is getting more intensive every 24 hours.
Conclusion
All the results prove that AOX1 promoter works properly.
Source
Mycobacterium Phage Bxb1
References
Roquet, N., Soleimany, A.P., Ferris, A.C., Aaronson, S. & Lu, T.K. Synthetic recombinase-based state machines in living cells. Science 353, aad8559 (2016).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 937
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]