Difference between revisions of "Part:BBa K3037002"
(→1) prove of DNA-binding ability of dCas9 via EMSA) |
(→3. Conclusions:) |
||
| (43 intermediate revisions by 4 users not shown) | |||
| Line 25: | Line 25: | ||
== Overview == | == Overview == | ||
| − | The [https://2019.igem.org/Team:TU_Dresden TU_Dresden 2019] team | + | The [https://2019.igem.org/Team:TU_Dresden TU_Dresden 2019] team designed this BioBrick in order to make a fusion protein [https://parts.igem.org/Part:BBa_K3037003 (BBa_K3037003)] with dCas9 in accordance to the [[Help:Assembly_standard_25|Freiburg RFC25 standard]]. [https://2019.igem.org/Team:TU_Dresden/Parts (more information)] |
| − | dCas9 was inserted into the pOCC97 [https://parts.igem.org/Part:BBa_K3037000 (BBa_K3037000)] | + | dCas9 was inserted into the pOCC97 vector [https://parts.igem.org/Part:BBa_K3037000 (BBa_K3037000)] for transformation and expression in <span style="font-style: italic;">Escherichia coli (E. coli)</span>. |
| − | There are many dCas9 | + | There are many dCas9 BioBricks already available, however, all of them are optimized for the expression in mammalian cells. This is the very first dCas9 BioBrick that has been codon optimized for expression in <span style="font-style: italic;">E. coli</span>. Therefore, we are adding a new scope of <i>in vitro</i> applications of Cas9, which is normally used <i>in vivo</i>, to the iGEM community. |
| − | + | ||
=== Biology === | === Biology === | ||
| − | dCas9 is | + | dCas9 is a mutant derived from Cas9. Together with CRISPR, which stores sequences of viral infections, Cas9 forms a primitive antiviral defense system in bacteria. Coupled with a guideRNA, Cas9 has the ability to bind to a specific sequence of DNA and cut it. However, in contrast to its natural counterpart, dCas9 does not longer have the endonuclease activity. That means it is only binding to specific DNA sequences without cutting them. [1] |
| − | The targeted sequence is determined by the guideRNA bound to the dCas9 protein. | + | The targeted sequence is determined by the guideRNA bound to the dCas9 protein. The binding reaction was incubated at 37°C for one hour. We can put this system to use by providing guideRNAs to locate to any target DNA sequence with a high specificity. |
== Characterization == | == Characterization == | ||
=== Overview === | === Overview === | ||
| − | |||
| − | |||
| − | |||
| − | ==== 1 | + | ===== 1. DNA-binding ability of dCas9 (BBa K3037002) ===== |
| + | The following characterization experiment was performed to prove the DNA-binding ability of dCas9 via an Electrophoretic Mobility Shift Assay (EMSA) (Performed with [https://parts.igem.org/Part:BBa_K3037005 BBa K3037005).] | ||
| − | + | ===== 2. DNA-binding ability of dCas9 in the Full Construct ([https://parts.igem.org/Part:BBa_K3037005 BBa K3037005])===== | |
| − | + | The same type of characterization experiment via EMSA was performed to prove the DNA-binding ability of the dCas9 contained in our Full Construct (see its registry page for more information regarding its structure [https://parts.igem.org/Part:BBa_K3037005 BBa K3037005]) | |
| − | + | ===== 3. Conclusions ===== | |
| − | + | === Experiment in Detail === | |
| − | |||
| − | + | ===== 1. DNA-binding ability of dCas9 (BBa K3037002)===== | |
| − | + | <b>a) Materials:</b> | |
| + | *100 ng of PCR amplified <span style="font-style: italic;">sry</span> gene | ||
| + | *200 ng of dCas9-GFP | ||
| + | *200 ng of guide RNA specifically targeting the amplified <span style="font-style: italic;">sry</span> gene | ||
| + | *1 x Reaction buffer - 20 mM Hepes buffer (pH 7.2) | ||
| + | ** 100 mM NaCl | ||
| + | ** 5 mM MgCl2 | ||
** 0.1 mM EDTA | ** 0.1 mM EDTA | ||
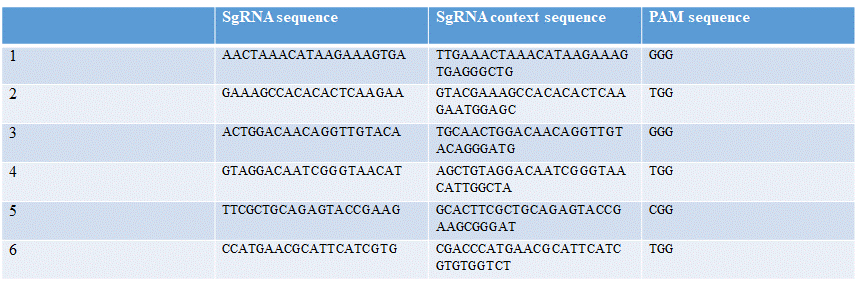
| − | Six different guide RNAs were designed for targeting different regions of <span style="font-style: italic;"> | + | Six different guide RNAs were designed for targeting different regions of <span style="font-style: italic;">sry</span> gene. Using the online tool Benchling and Fasta sequence of <span style="font-style: italic;">sry</span> gene (Table 1). |
1: AACTAAACATAAGAAAGTGA | 1: AACTAAACATAAGAAAGTGA | ||
| Line 80: | Line 82: | ||
6: CCATGAACGCATTCATCGTG | 6: CCATGAACGCATTCATCGTG | ||
| − | [[File:T--TU_Dresden--EMSA_Primers_BBa_K3037005.png|center|400px|thumb|left| | + | [[File:T--TU_Dresden--EMSA_Primers_BBa_K3037005.png|center|400px|thumb|left|Table 1: Overview of different guide RNAs with the context of the sequence and the PAM sequence]] |
| − | <b> | + | <b>b) Methods:</b> |
| − | 1. We wanted to check if the overall efficiency of mobility shift increases when combinations of guide RNAs are | + | 1. We wanted to check if the overall efficiency of mobility shift increases when combinations of guide RNAs are used. |
| − | 2. Guide RNA, dCas9-GFP and <span style="font-style: italic;"> | + | 2. Guide RNA, dCas9-GFP and <span style="font-style: italic;">sry</span> gene were incubated in reaction buffer (respective amounts mentioned in the materials section) for 37 °C for 1 hour. |
| − | 3. Post incubation, they were mixed with loading dye without SDS, 20 % glycerol in Orange G dye and loaded onto 4-20 % gradient acrylamide- TBE precast gel. | + | 3. Post incubation, they were mixed with loading dye without SDS, 20 % glycerol in Orange G dye and loaded onto a 4-20 % gradient acrylamide- TBE precast gel. Two gels were run for 2 and 3 hours at 75 V in 1 x TBE buffer. |
4. Gel was then stained using EtBR with 1:20000 dilution in 1x TBE for 10 minutes. | 4. Gel was then stained using EtBR with 1:20000 dilution in 1x TBE for 10 minutes. | ||
| + | <b> Results and Discussion </b> | ||
| + | <b> (i) Two hours gel:</b> | ||
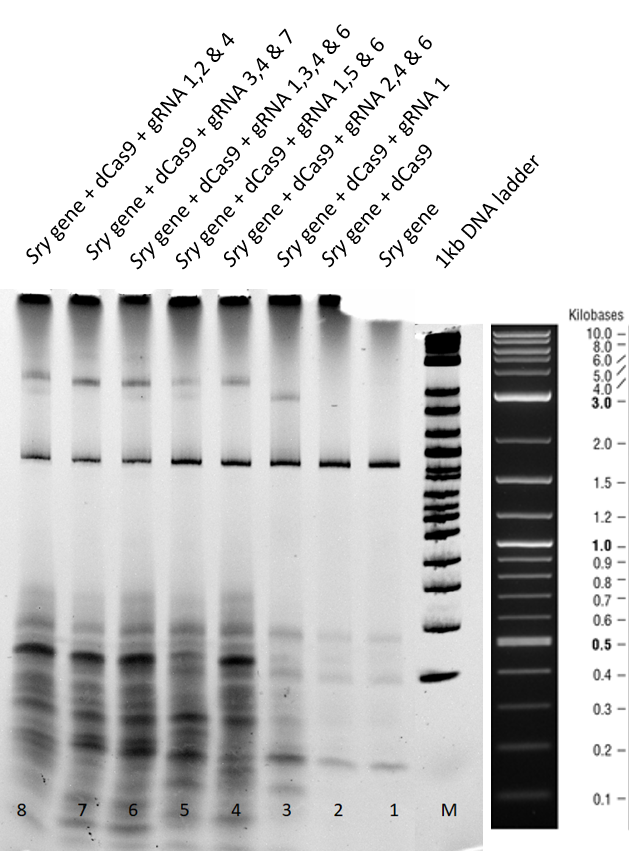
| − | + | [[File:T--TU_Dresden--EMSA1_BBa_K3037005.png|center|400px|thumb|left|Figure 1: Electrophoretic Mobility Shift Assay (EMSA) after a two hour run using dCas9 with different guideRNAs]] | |
| − | + | Lane 1+2 - There is a clear <span style="font-style: italic;">sry</span> band at 800 base pairs and when the <span style="font-style: italic;">sry</span> gene is incubated with only dCas9 without guideRNA. Over all, no shift is observed. | |
| − | + | Lane 3 - When guideRNA 1 was incubated with the dCas9 DNA reaction mix, we saw a shift in the mobility, this is because of the protein DNA interaction and this binding is hindering the gene mobility. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | Lane 3 - When | + | |
Lanes 5,6,7,8 and 9 – Different combinations of guide RNAs were used. From lane 7 and 8 we see the highest mobility shift. | Lanes 5,6,7,8 and 9 – Different combinations of guide RNAs were used. From lane 7 and 8 we see the highest mobility shift. | ||
| − | From the electromobility shift assay performed above, we conclude that our expressed dCas9-GFP protein is functional and is able to successfully bind to gene with the help of appropriate | + | From the electromobility shift assay performed above, we conclude that our expressed dCas9-GFP protein is functional and is able to successfully bind to gene with the help of appropriate guideRNAs. |
| − | <b> | + | <b> (ii) Three hours gel:</b> |
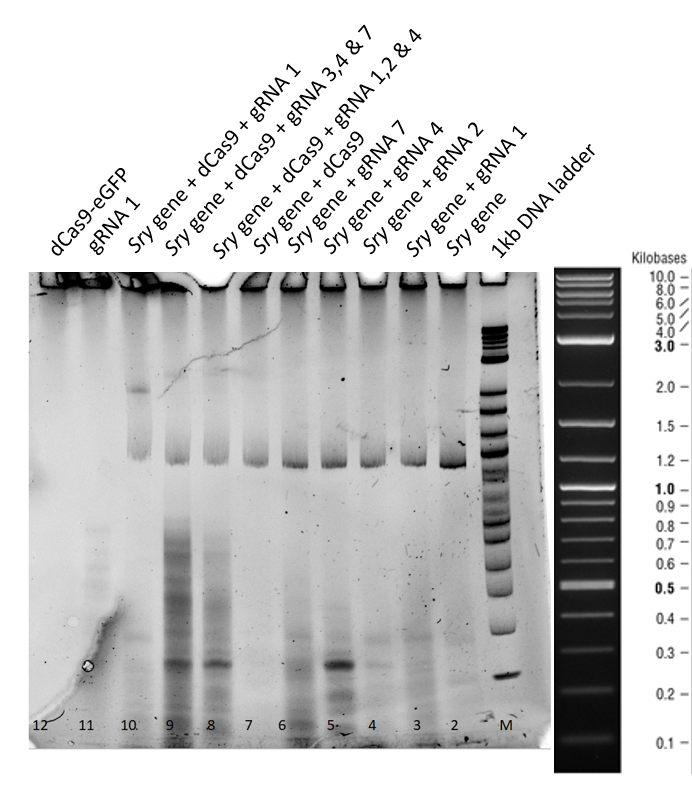
| − | [[File:T--TU_Dresden--EMSA2_BBa_K3037005.png|center|400px|thumb|left]] | + | [[File:T--TU_Dresden--EMSA2_BBa_K3037005.png|center|400px|thumb|left|Figure 2: Electrophoretic Mobility Shift Assay (EMSA) after a three hour run of the dCas9 with different guideRNAs]] |
| − | This second gel was run | + | This second gel was run longer in order to get rid of all the secondary structures derived from residual RNA fragments. |
| − | From lane 3 to 7, no difference in the mobility of | + | From lane 3 to 7, no difference in the mobility of <i>sry</i> gene can be seen when only guideRNA is added to the reaction mix. |
| − | In Lane 8, 9 and 10 a mobility shift of the gene can be | + | In Lane 8, 9 and 10 a mobility shift of the gene can be observed and in lane 11, when only guideRNA was loaded no bands were obtained. |
| − | In lane 12, dCas9 is in stacking part of gel, owing to higher molecular weight. | + | In lane 12, dCas9 is in the stacking part of gel, owing to higher molecular weight. |
| − | |||
| − | |||
| − | - dCas9 on its own is unable to bind to <span style="font-style: italic;"> | + | |
| + | ===== 2. DNA-binding ability of dCas9 in the Full Construct ([https://parts.igem.org/Part:BBa_K3037005 BBa K3037005])===== | ||
| + | |||
| + | Once again, the DNA binding activity of dCas9 was verified via EMSA. This time, the dCas9 is a single contained in our Full Construct. In Figure 3, it can be clearly seen how at very high concentrations of expressed Full Construct, our dCas9 is able to completely bind to the <i>sry</i> gene, fully hindering its mobility through the gel (red box). | ||
| + | |||
| + | [[File:T--TU_Dresden--emsa1.png|center|400px|thumb|none|Figure 3: Proof of the binding of our Full Construct (with its dCas9) to the DNA sequence of interest (SRY gene in this experiment).]] | ||
| + | |||
| + | ===== 3. Conclusions: ===== | ||
| + | |||
| + | - We have a functional dCas9 expressed, which is able to bind successfully to <span style="font-style: italic;">sry</span> gene with the help of specific guideRNAs. | ||
| + | |||
| + | - dCas9 on its own is unable to bind to <span style="font-style: italic;">sry</span> gene, proving that for binding at least one appropriate guideRNA is required. | ||
| − | - | + | - GuideRNAs on their own are unable to cause a mobility shift of the <span style="font-style: italic;">sry</span> gene. |
| − | |||
| − | + | In order to determine the lowest concentration at which our expressed Full Construct causes the pull of the gene, different concentrations were loaded on the gradient TBE acrylamide gel. We found that at approximately 1.28 ug the dCas9 is able to bind and therefore, pull up the DNA. Additionally, at 8.56 ug we can see a very clear shift of the DNA, since it can be seen in the region marked with a red box in Figure 4. | |
| + | [[File:T--TU_Dresden--emsa2.png|center|600px|thumb|none|Figure 4: Determination of the lowest concentration of the Full Construct (with dCas9) needed to bind and pull DNA.]] | ||
| + | |||
| + | == Sequence == | ||
| + | |||
| + | <partinfo>BBa_K3037002 SequenceAndFeatures</partinfo> | ||
| − | + | == Design Notes == | |
| − | In the middle of the coding sequence there was an EcoRI site | + | In the middle of the coding sequence there was an EcoRI site. Therefore, we performed a site directed mutagenesis PCR with the following primers to erase the forbidden restriction site: |
| − | Primer 1: | + | Primer 1: GATCGAATTCGCGGCCGCTTCTAGATAAGGAGGTCAAAAATGgccggcGATAAGAAATACTCAATAGGC |
| − | Primer 2: | + | Primer 2: CATAATAAGGAATACGAAAAGTCAAG |
| − | Primer 3: | + | Primer 3: CATAATAAGGAATACGAAAAGTCAAG |
| − | Primer 4: | + | Primer 4: GATCTCTGCAGCGGCCGCTACTAGTATTAACCGGTGTCACCTCCTAGCTGACTCAAATC |
[[File:DCas9 site directed mutagenesis.jpeg|center|400px|thumb|none|dCas9 site directed mutagenesis]] | [[File:DCas9 site directed mutagenesis.jpeg|center|400px|thumb|none|dCas9 site directed mutagenesis]] | ||
| − | The primers used to | + | The primers used to adapt this BioBrick to the [[Help:Assembly_standard_25|Freiburg RFC25 standard]] were the following ones: |
Prefix: GAATTCGCGGCCGCTTCTAGATAAGGAGGTCAAAAATGgccggc | Prefix: GAATTCGCGGCCGCTTCTAGATAAGGAGGTCAAAAATGgccggc | ||
| Line 155: | Line 168: | ||
Find more information in [https://2019.igem.org/Team:TU_Dresden here] | Find more information in [https://2019.igem.org/Team:TU_Dresden here] | ||
| − | + | == References == | |
| + | [1] Whinn KS, Kaur G, Lewis JS, et al. (2019) Nuclease dead Cas9 is a programmable roadblock for DNA replication. <i>Sci Rep.</i> Sep 16;9(1):13292 | ||
Latest revision as of 03:10, 22 October 2019
dead CRISPR Associated Protein (dCas9)
| dCas9 | |
|---|---|
| Function | Expression |
| Use in | Escherichia coli |
| RFC standard | Freiburg RFC25 standard |
| Backbone | pSB1C3 |
| Experimental Backbone | pOCC97 |
| Submitted by | Team: TU_Dresden 2019 |
Contents
Overview
The TU_Dresden 2019 team designed this BioBrick in order to make a fusion protein (BBa_K3037003) with dCas9 in accordance to the Freiburg RFC25 standard. (more information)
dCas9 was inserted into the pOCC97 vector (BBa_K3037000) for transformation and expression in Escherichia coli (E. coli).
There are many dCas9 BioBricks already available, however, all of them are optimized for the expression in mammalian cells. This is the very first dCas9 BioBrick that has been codon optimized for expression in E. coli. Therefore, we are adding a new scope of in vitro applications of Cas9, which is normally used in vivo, to the iGEM community.
Biology
dCas9 is a mutant derived from Cas9. Together with CRISPR, which stores sequences of viral infections, Cas9 forms a primitive antiviral defense system in bacteria. Coupled with a guideRNA, Cas9 has the ability to bind to a specific sequence of DNA and cut it. However, in contrast to its natural counterpart, dCas9 does not longer have the endonuclease activity. That means it is only binding to specific DNA sequences without cutting them. [1]
The targeted sequence is determined by the guideRNA bound to the dCas9 protein. The binding reaction was incubated at 37°C for one hour. We can put this system to use by providing guideRNAs to locate to any target DNA sequence with a high specificity.
Characterization
Overview
1. DNA-binding ability of dCas9 (BBa K3037002)
The following characterization experiment was performed to prove the DNA-binding ability of dCas9 via an Electrophoretic Mobility Shift Assay (EMSA) (Performed with BBa K3037005).
2. DNA-binding ability of dCas9 in the Full Construct (BBa K3037005)
The same type of characterization experiment via EMSA was performed to prove the DNA-binding ability of the dCas9 contained in our Full Construct (see its registry page for more information regarding its structure BBa K3037005)
3. Conclusions
Experiment in Detail
1. DNA-binding ability of dCas9 (BBa K3037002)
a) Materials:
- 100 ng of PCR amplified sry gene
- 200 ng of dCas9-GFP
- 200 ng of guide RNA specifically targeting the amplified sry gene
- 1 x Reaction buffer - 20 mM Hepes buffer (pH 7.2)
- 100 mM NaCl
- 5 mM MgCl2
- 0.1 mM EDTA
Six different guide RNAs were designed for targeting different regions of sry gene. Using the online tool Benchling and Fasta sequence of sry gene (Table 1).
1: AACTAAACATAAGAAAGTGA
2: GAAAGCCACACACTCAAGAA
3: ACTGGACAACAGGTTGTACA
4: GTAGGACAATCGGGTAACAT
5: TTCGCTGCAGAGTACCGAAG
6: CCATGAACGCATTCATCGTG
b) Methods:
1. We wanted to check if the overall efficiency of mobility shift increases when combinations of guide RNAs are used.
2. Guide RNA, dCas9-GFP and sry gene were incubated in reaction buffer (respective amounts mentioned in the materials section) for 37 °C for 1 hour.
3. Post incubation, they were mixed with loading dye without SDS, 20 % glycerol in Orange G dye and loaded onto a 4-20 % gradient acrylamide- TBE precast gel. Two gels were run for 2 and 3 hours at 75 V in 1 x TBE buffer.
4. Gel was then stained using EtBR with 1:20000 dilution in 1x TBE for 10 minutes.
Results and Discussion (i) Two hours gel:
Lane 1+2 - There is a clear sry band at 800 base pairs and when the sry gene is incubated with only dCas9 without guideRNA. Over all, no shift is observed.
Lane 3 - When guideRNA 1 was incubated with the dCas9 DNA reaction mix, we saw a shift in the mobility, this is because of the protein DNA interaction and this binding is hindering the gene mobility.
Lanes 5,6,7,8 and 9 – Different combinations of guide RNAs were used. From lane 7 and 8 we see the highest mobility shift.
From the electromobility shift assay performed above, we conclude that our expressed dCas9-GFP protein is functional and is able to successfully bind to gene with the help of appropriate guideRNAs.
(ii) Three hours gel:
This second gel was run longer in order to get rid of all the secondary structures derived from residual RNA fragments.
From lane 3 to 7, no difference in the mobility of sry gene can be seen when only guideRNA is added to the reaction mix.
In Lane 8, 9 and 10 a mobility shift of the gene can be observed and in lane 11, when only guideRNA was loaded no bands were obtained.
In lane 12, dCas9 is in the stacking part of gel, owing to higher molecular weight.
2. DNA-binding ability of dCas9 in the Full Construct (BBa K3037005)
Once again, the DNA binding activity of dCas9 was verified via EMSA. This time, the dCas9 is a single contained in our Full Construct. In Figure 3, it can be clearly seen how at very high concentrations of expressed Full Construct, our dCas9 is able to completely bind to the sry gene, fully hindering its mobility through the gel (red box).
3. Conclusions:
- We have a functional dCas9 expressed, which is able to bind successfully to sry gene with the help of specific guideRNAs.
- dCas9 on its own is unable to bind to sry gene, proving that for binding at least one appropriate guideRNA is required.
- GuideRNAs on their own are unable to cause a mobility shift of the sry gene.
In order to determine the lowest concentration at which our expressed Full Construct causes the pull of the gene, different concentrations were loaded on the gradient TBE acrylamide gel. We found that at approximately 1.28 ug the dCas9 is able to bind and therefore, pull up the DNA. Additionally, at 8.56 ug we can see a very clear shift of the DNA, since it can be seen in the region marked with a red box in Figure 4.
Sequence
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 1096
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 3375
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Design Notes
In the middle of the coding sequence there was an EcoRI site. Therefore, we performed a site directed mutagenesis PCR with the following primers to erase the forbidden restriction site:
Primer 1: GATCGAATTCGCGGCCGCTTCTAGATAAGGAGGTCAAAAATGgccggcGATAAGAAATACTCAATAGGC
Primer 2: CATAATAAGGAATACGAAAAGTCAAG
Primer 3: CATAATAAGGAATACGAAAAGTCAAG
Primer 4: GATCTCTGCAGCGGCCGCTACTAGTATTAACCGGTGTCACCTCCTAGCTGACTCAAATC
The primers used to adapt this BioBrick to the Freiburg RFC25 standard were the following ones:
Prefix: GAATTCGCGGCCGCTTCTAGATAAGGAGGTCAAAAATGgccggc
Suffix: accggttaaTACTAGTAGCGGCCGCTGCAG
Find more information in here
References
[1] Whinn KS, Kaur G, Lewis JS, et al. (2019) Nuclease dead Cas9 is a programmable roadblock for DNA replication. Sci Rep. Sep 16;9(1):13292