Part:BBa_K3037000
pOCC97 plasmid backbone for expression (optimized)
| pOCC97 | |
|---|---|
| Function | Backbone for expression |
| Use in | Escherichia coli pRARE T7 |
| RFC standard | RFC 10 |
| Submitted by | Team: TU_Dresden 2019 |
Contents
Overview
The TU Dresden 2019 team designed this BioBrick in order to express its fusion proteins (more information).
Features:
- IPTG inducible promoter
- LacO promoter and LacI inhibitor
- Kanamycin resistance
- Needs T7 RNA polymerase (a viral RNA polymerase for high expression)
- Contains SP6 site which is a commonly used primer site for sequencing the inserted part
- Plasmid is optimized for E. coli use
- For optimal results use BBa_K3037000 in combination with a pRARE plasmid, carried by the used E. coli strain
- In case you use pRARE, you need to supplement your media with two antibiotics - Kanamycin (Kan) and Chloramphenicol (Cm)
- Alternatively, use a strain which can express T7 from its genome, regular E. coli strains do not express T7 by default
Very well established expression plasmid for recombinant proteins in E. coli:
getting any BioBrick you need, from cloning to expression in just 24 hours!
Biology
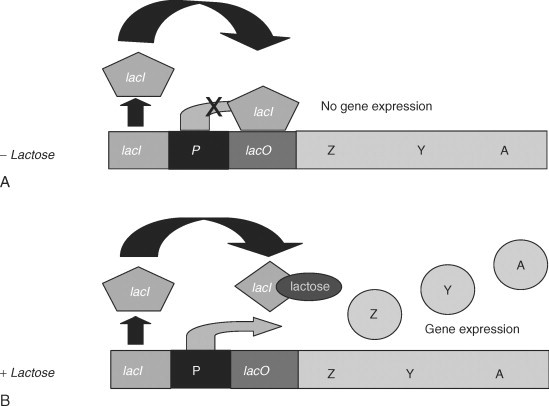
The lactose operon (lac operon) is a polycistronic bacterial operon that encodes the genes of lactose metabolism. It consists of three structural genes: a promoter, an operator and a terminator (Summarized in Figure 1). A bacterial cell synthesizes enzymes involved in lactose metabolism only under two conditions: in the presence of lactose and/or when the cells are lacking glucose. [1]
The regulation of the lac operon occurs according to the principle of negative feedback: the more lactose is present in the environment, the more enzymes for its catabolism are synthesized (positive direct connection); the more enzymes are present in a cell, the less lactose remains, and finally, the less lactose in the environment, the less enzyme is produced (double negative feedback). [2]
In the absence of lactose in the cell, or at a low concentrations, the repressor protein reversibly binds to the operator region and inhibits transcription. The reporter protein is a product of the LacI monocistronic operon. In the absence of lactose in the cell, enzymes for lactose metabolism are not synthesized. Besides, if the glucose concentration in the cell is sufficient to maintain metabolism, activation of the lactose operon also does not occur. The promoter sequence of the lactose operon is weak, therefore, even in the absence of a repressor protein in the operator site, transcription is practically not initiated.
When the concentration of glucose in the cell decreases, the enzyme adenylate cyclase is activated. Glucose is an inhibitor of this enzyme and activates phosphodiesterase, which catalyzes the conversion of the cAMP molecule to AMP. Adenylate cyclase catalyzes the conversion of ATP to the cyclic form - cAMP. cAMP binds to a catabolism activating protein (CAP), and a complex is formed that interacts with the promoter of the lactose operon. It changes its conformation, and increases the affinity of RNA polymerase for this site. In the presence of lactose, expression of the operon genes occurs. [2]
Characterization
Outline
1) Expression of different proteins: monitoring growth
2) Expression of proteins with our backbone before and after optimization
3) SDS-PAGEs for the expression assay over the time of Full Construct (BBa_K3037003)
4) Image analysis of the expression in the SDS-PAGEs with ImageJ
Experiments in Detail
1) Expression of different proteins: monitoring growth
To evaluate the impact on the metabolic burden of over-expressing proteins, we tested different constructs cloned into our backbone. For this we used: HRP (BBa_K3037007) and our full construct (BBa_K3037003). After cloning, the constructs were expressed in E. coli pRARE T7 and we monitored growth over time (Figure 2). Induction of the system was performed after 165 min with 1 mM IPTG. Overall, the results prove that when expressed, none of our proteins inhibit growth.
2) Expression of proteins with our backbone before and after optimization
To use this backbone as convenient expression vector in iGEM, we had to include the Prefix and Suffix of the BioBrick Assembly. Subsequentially, we compared the expression of the original vector we recieved, pOCC97, to our optimized version for iGEM, BBa_K3730000.
We found that the original plasmid (= non-optimized) had a XbaI site, which we used to insert our BioBrick BBa_K3037003. This ilegal restriction site was later removed with an overhang PCR. The original XbaI restriction site was positioned downstream of the T7 polymerase promoter and upstream the RBS sequence of the plasmid. This way only BioBricks that already has a RBS fused to them could be expressed. Since we were using the RFC25 standard of Freiburg for our fusion proteins, the inserted protein contained already its own RBS in the Prefix.
However, we experienced on our own how difficult it is to add such a small sequence, as an RBS, to our other constructs. Therefore we redesigned the plasmid to be ready for expression in a single digestion+ligation reaction. We removed the XbaI restriction site and included a Prefix and Suffix of the RFC 10 standard after the RBS of the plasmid (=optimized).
We used the BioBrick assembly method to insert our BioBrick BBa_K3037003, which also has its own RBS due to the RFC25 standard.
In Figure 3, we show the expression of the protein BBa_K3037003 using both plasmids optimized (left) and non-optimized (right) and also different IPTG concentrations for induction and temperature.
The comparison of the growth curves shows that, the new plasmid adapted to the RFC 10 standard did not affect the growth, as it shows similar behavior compared to the original one (Figure 4).
3) SDS-PAGEs of the expression assays of the full construct (BBa_K3037003)
Down below follow several SDS-PAGEs of loaded crude cell extract (all normalized to an OD of 0.5) harvested at different time points pre- and post-induction. Used IPTG concentrations are indicated. Arrows indicate predicted size of the protein of interest.
Comparison of the expression of MBP-HRP (BBa_K3037008) and Full Construct (BBa_3037003)
Expression of full construct in pOCC97 not optimized at 18ÂșC
Expression of Full Construct in pOCC97 at 37ÂșC
Expression of Full Construct in pOCC97 optimized
4) Image analysis of the expression in the SDS-PAGEs with ImageJ
The previously shown SDS-pages were then further analysed by using the software ImageJ to correct for loading differences and be able to draw conclusions about the best conditions to express the Full Construct in pOCC97.
Temperature and IPTG induction dependence of the optimized pOCC97
Temperature and IPTG induction dependence of the not optimized pOCC97
Comparison between optimized and not optimized pOCC97
Conclusion
Based on this analysis, it can be concluded that optimal conditions for the expression of our fusion protein, BBa_3037003, is an overnight expression at 18ÂșC and inducing with 0.5 mM IPTG.
We are proud to say that our optimized pOCC97 shows an increased expression and robustness under various conditions tested.
Sequence
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Plasmid lacks a prefix.
Plasmid lacks a suffix.
Illegal EcoRI site found at 5283
Illegal SpeI site found at 2
Illegal PstI site found at 16
Illegal NotI site found at 9
Illegal NotI site found at 5289 - 21INCOMPATIBLE WITH RFC[21]Plasmid lacks a prefix.
Plasmid lacks a suffix.
Illegal EcoRI site found at 5283
Illegal BglII site found at 5169 - 23INCOMPATIBLE WITH RFC[23]Illegal prefix found at 5283
Illegal suffix found at 2 - 25INCOMPATIBLE WITH RFC[25]Illegal prefix found at 5283
Plasmid lacks a suffix.
Illegal XbaI site found at 5298
Illegal SpeI site found at 2
Illegal PstI site found at 16
Illegal NgoMIV site found at 342
Illegal NgoMIV site found at 3389
Illegal NgoMIV site found at 3549
Illegal NgoMIV site found at 5137 - 1000INCOMPATIBLE WITH RFC[1000]Plasmid lacks a prefix.
Plasmid lacks a suffix.
Illegal SapI.rc site found at 2468
Design Notes
This BioBrick was designed to fit the RFC 10 standard using the primers:
Forward: tactagtagcggccgctgcagCCGTTATAGAAGCTTGAGTATT
Reverse: gaattcgcggccgcttctagagGCCCATGGATATATCTCCTTCT
References
1. Jacob F; Monod J. Genetic regulatory mechanisms in the synthesis of proteins, J Mol Biol. journal, 1961, vol. 3: p. 318â356.
2. J. Parker, Encyclopedia of Genetics, 2001
3. Klaus I. Matthaei, in Handbook of Stem Cells, 2004| None |