Difference between revisions of "Part:BBa K3254000"
(→Experimental Setup) |
|||
| (4 intermediate revisions by 3 users not shown) | |||
| Line 10: | Line 10: | ||
===Experimental Setup=== | ===Experimental Setup=== | ||
| − | *Genetic design principle of the experimental group | + | *Genetic design principle of the experimental group was described on the page of [[Part:BBa_K3254010|BBa_K3254010]]. |
*A P15A-AmpR plasmid was co-transfered into the E.coli DH5α host cell with the reporter plasmid containing this part as the negative control. | *A P15A-AmpR plasmid was co-transfered into the E.coli DH5α host cell with the reporter plasmid containing this part as the negative control. | ||
*Single colonies were selected from the experimental LB-agar plate and negative control LB-agar plate, then inoculated into EP tubes with 500 μL M9 supplemented medium containing 500 μM IPTG for overnight growth at 37 °C and 200 rpm. | *Single colonies were selected from the experimental LB-agar plate and negative control LB-agar plate, then inoculated into EP tubes with 500 μL M9 supplemented medium containing 500 μM IPTG for overnight growth at 37 °C and 200 rpm. | ||
*Tubes were centrifuged at 10000g for 1 min. Then observed the GFP fluorescence of the cell precipitations under blue light. | *Tubes were centrifuged at 10000g for 1 min. Then observed the GFP fluorescence of the cell precipitations under blue light. | ||
| + | *M9 medium (supplemented): 6.8 g/L Na<sub>2</sub>HPO<sub>4</sub>, 3 g/L KH<sub>2</sub>PO<sub>4</sub>, 0.5 g/L NaCl, 1 g/L NH<sub>4</sub>Cl, 0.34 g/L thiamine, 0.2% casamino acids, 0.4% glucose, 2mM MgSO<sub>4</sub>, and 100 μM CaCl<sub>2</sub>. | ||
===Results=== | ===Results=== | ||
| Line 26: | Line 27: | ||
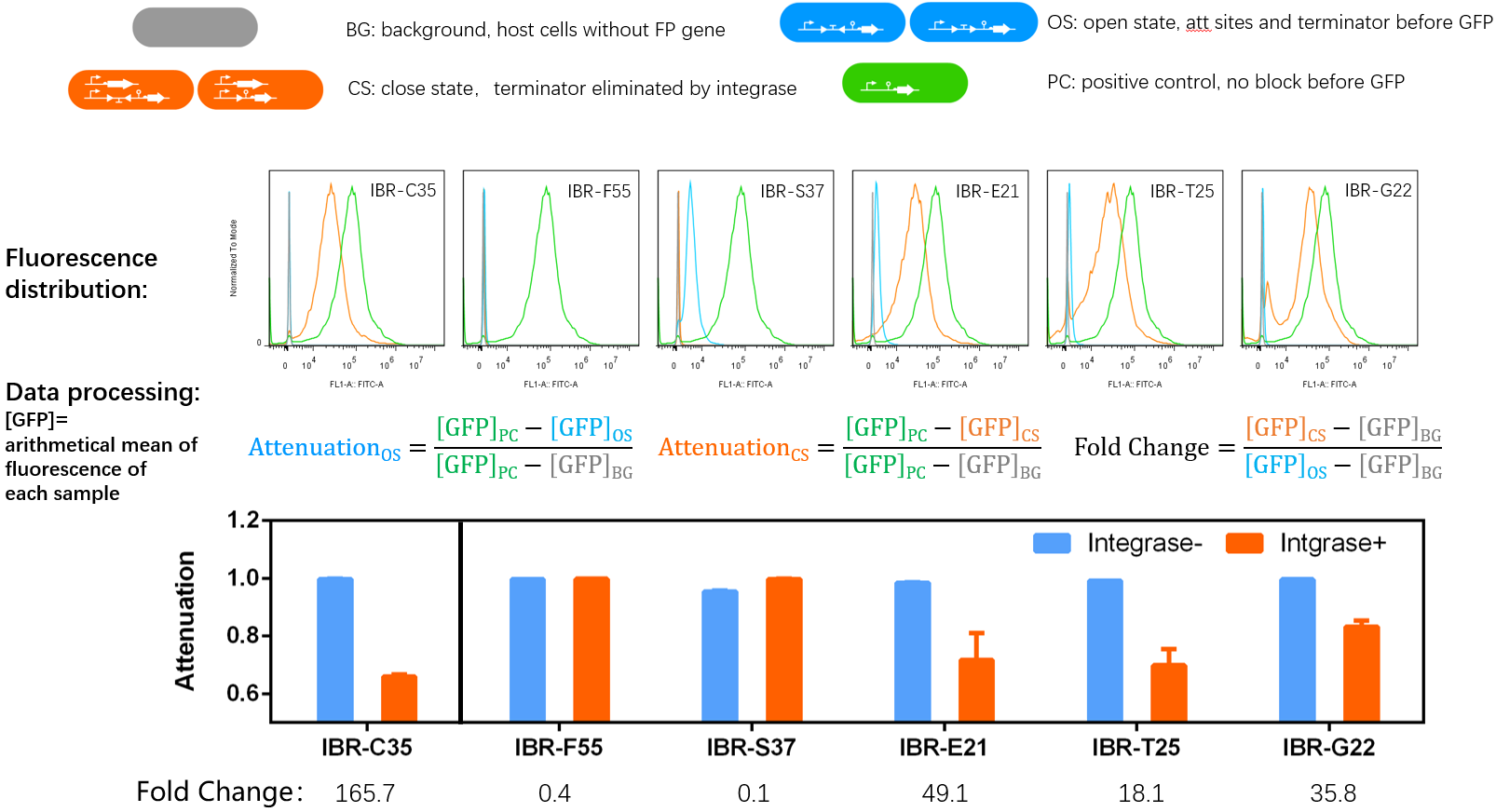
*Bacteria harboring the circuits (see the top part of the result image) first inoculated from single colonies into a flat-bottom 96-well plate for overnight growth. Then, the cell cultures were diluted 1000-fold with M9 supplemented medium with 500 μM IPTG inducer and growth for another 20 hours. All incubations were carried out using a Digital Thermostatic Shaker maintained at 37 °C and 1000 rpm, using flat-bottom 96-well plates sealed with sealing film. Finally, 3-μL samples each culture were transferred to a new 96-well plate containing 200 μL per well of PBS supplemented with 2 mg/mL kanamycin. | *Bacteria harboring the circuits (see the top part of the result image) first inoculated from single colonies into a flat-bottom 96-well plate for overnight growth. Then, the cell cultures were diluted 1000-fold with M9 supplemented medium with 500 μM IPTG inducer and growth for another 20 hours. All incubations were carried out using a Digital Thermostatic Shaker maintained at 37 °C and 1000 rpm, using flat-bottom 96-well plates sealed with sealing film. Finally, 3-μL samples each culture were transferred to a new 96-well plate containing 200 μL per well of PBS supplemented with 2 mg/mL kanamycin. | ||
*The fluorescence distribution of each sample was assayed using a flow cytometry. The arithmetical mean of each sample was determined using FlowJo software. | *The fluorescence distribution of each sample was assayed using a flow cytometry. The arithmetical mean of each sample was determined using FlowJo software. | ||
| − | *The principle of data processing | + | *The principle of data processing was shown on the result image. |
===Results=== | ===Results=== | ||
| Line 49: | Line 50: | ||
===Genetic Design=== | ===Genetic Design=== | ||
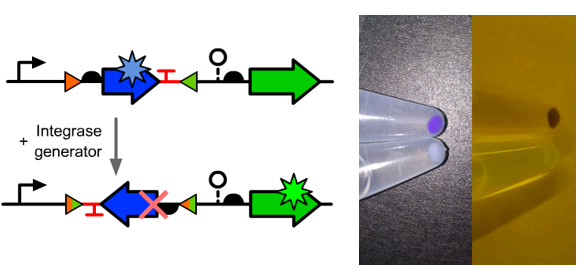
| − | *The composition and principle of the experimental system | + | *The composition and principle of the experimental system were indicated below. |
[[File:T--GENAS_China--Flip_with_backbone.PNG|200px|thumb|left|The composition and principle of the experimental system]] | [[File:T--GENAS_China--Flip_with_backbone.PNG|200px|thumb|left|The composition and principle of the experimental system]] | ||
===Experimental Setup=== | ===Experimental Setup=== | ||
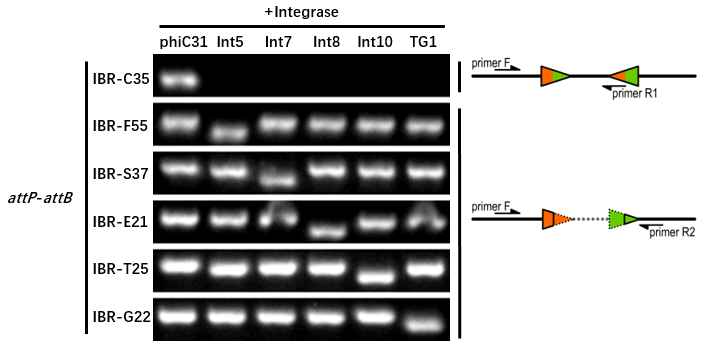
| − | The reporter plasmid | + | The reporter plasmid containing this part were co-transferred into E.coli DH5α host with 6 integrase generator plasmids. |
| − | Then single colonies were inoculated into M9 supplemented medium for overnight growth. Then, the cell cultures were diluted 1000-fold with M9 supplemented medium with 500 μM IPTG inducer and growth for another 20 hours. All incubations were carried out using a Digital Thermostatic Shaker maintained at 37 °C and 1000 rpm, using flat-bottom 96-well plates sealed with sealing film. Finally, | + | Then single colonies were inoculated into M9 supplemented medium for overnight growth. Then, the cell cultures were diluted 1000-fold with M9 supplemented medium with 500 μM IPTG inducer and growth for another 20 hours. All incubations were carried out using a Digital Thermostatic Shaker maintained at 37 °C and 1000 rpm, using flat-bottom 96-well plates sealed with sealing film. Finally, the cultures were sampled for genotype PCR testing. |
The principle of genotype identification was shown on the right of results image. | The principle of genotype identification was shown on the right of results image. | ||
| Line 66: | Line 67: | ||
| − | + | 1111111111111111 | |
| − | + | ||
Latest revision as of 06:38, 20 September 2022
phiC31attB-BsaI sites-terminator-phiC31attP(r)
This part is an improvement for the part BBa_K2243031. It can be placed between a promoter and a translational unit part and works as a normally open (NO) switch for the downstreamed gene, and switch to ON state by flipping the unidirectional terminator ECK120034435 between the att sites when it reacted with phiC31 integrase BBa_K1039012. In this improved version, two BsaI restriction site were added between attB site and terminator. As a result, it can work as a normally closed (NC) switch for the gene which was inserted between the two BsaI site and switch to OFF state when it flipped.
Usage and Biology
Visual Result as a Normally Open Switch
- We conducted a simple test to see if our design met the expection.
Experimental Setup
- Genetic design principle of the experimental group was described on the page of BBa_K3254010.
- A P15A-AmpR plasmid was co-transfered into the E.coli DH5α host cell with the reporter plasmid containing this part as the negative control.
- Single colonies were selected from the experimental LB-agar plate and negative control LB-agar plate, then inoculated into EP tubes with 500 μL M9 supplemented medium containing 500 μM IPTG for overnight growth at 37 °C and 200 rpm.
- Tubes were centrifuged at 10000g for 1 min. Then observed the GFP fluorescence of the cell precipitations under blue light.
- M9 medium (supplemented): 6.8 g/L Na2HPO4, 3 g/L KH2PO4, 0.5 g/L NaCl, 1 g/L NH4Cl, 0.34 g/L thiamine, 0.2% casamino acids, 0.4% glucose, 2mM MgSO4, and 100 μM CaCl2.
Results
- IBR-C35/F55/S37/E21/T25/G22 indicated the experimental systems for phiC31/Int5/Int7/Int8/Int10/TG1 respctively.
- We observed the GFP fluorescence from the experimental tube as expected.
Quantitative Characterizaion of the Normally Open Switch
Experimental Setup
- Bacteria harboring the circuits (see the top part of the result image) first inoculated from single colonies into a flat-bottom 96-well plate for overnight growth. Then, the cell cultures were diluted 1000-fold with M9 supplemented medium with 500 μM IPTG inducer and growth for another 20 hours. All incubations were carried out using a Digital Thermostatic Shaker maintained at 37 °C and 1000 rpm, using flat-bottom 96-well plates sealed with sealing film. Finally, 3-μL samples each culture were transferred to a new 96-well plate containing 200 μL per well of PBS supplemented with 2 mg/mL kanamycin.
- The fluorescence distribution of each sample was assayed using a flow cytometry. The arithmetical mean of each sample was determined using FlowJo software.
- The principle of data processing was shown on the result image.
Results
- IBR-C35/F55/S37/E21/T25/G22 indicated the experimental systems for phiC31/Int5/Int7/Int8/Int10/TG1 respctively.
- Compared to other parts, this part performed well.
Visual Results as a Normally Closed Switch and Toggle Switch
- We inserted an amilCP translational unit between the two BsaI sites.
- Other experiment setup were the same with "Visual Result as a Normally Closed Switch".
Results
- The normally open switch function well though a fade blue color can be observed from the cell precipitations which might due to the incomplete diluted amilCP protein or an unexpected backward promoter.
- At the same time, the downstreamed GFP wasn't expression well which might due to the potential attenuation signal in the reversed amilCP sequence.
Thermodynamic Characterization
See the related information on the page of BBa_K3254010.
Orthogonality Characterization
Genetic Design
- The composition and principle of the experimental system were indicated below.
Experimental Setup
The reporter plasmid containing this part were co-transferred into E.coli DH5α host with 6 integrase generator plasmids. Then single colonies were inoculated into M9 supplemented medium for overnight growth. Then, the cell cultures were diluted 1000-fold with M9 supplemented medium with 500 μM IPTG inducer and growth for another 20 hours. All incubations were carried out using a Digital Thermostatic Shaker maintained at 37 °C and 1000 rpm, using flat-bottom 96-well plates sealed with sealing film. Finally, the cultures were sampled for genotype PCR testing. The principle of genotype identification was shown on the right of results image.
Results
- IBR-C35 was the plasmid containing this part.
- The result indicates that this part can only be recombined by phiC31 integrase.
- The sequences after recombination are tgcgGGTGCCAGGGCGTGCCCTTGAGTTCTCTCAGTTGGGGG (attL) and ggagtaCGCGCCCGGGGAGCCCAAAGGTTACCCCAGTTGGGGcac (attR).
1111111111111111
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 59
Illegal BsaI.rc site found at 47