Difference between revisions of "Part:BBa K2598009"
| (One intermediate revision by the same user not shown) | |||
| Line 38: | Line 38: | ||
<b>Figure 5</b> shows results of gel electrophoresis of various parts after PCR with primer VF2\VR. The distance between primer binding sites and both ends of the parts are approximately 150 bp, thus rendering the product about 300 bp longer. The picture is edited to show a more compact photo. | <b>Figure 5</b> shows results of gel electrophoresis of various parts after PCR with primer VF2\VR. The distance between primer binding sites and both ends of the parts are approximately 150 bp, thus rendering the product about 300 bp longer. The picture is edited to show a more compact photo. | ||
<div>[[File:T—UCAS-China—figure8 2018.png|700px|thumb|center|<b>Figure 5:</b> Results of gel electrophoresis of various parts after PCR with primer VF2\VR]]</div> | <div>[[File:T—UCAS-China—figure8 2018.png|700px|thumb|center|<b>Figure 5:</b> Results of gel electrophoresis of various parts after PCR with primer VF2\VR]]</div> | ||
| + | |||
| + | |||
| + | <div style="color:black;font-size:25px">Characterization from SDU_CHINA 2019</div> | ||
<b>Figure 6/7</b> shows the date of the quantitative experimental characterization from SDU_CHINA 2019. Cells were cultured in the LB medium for 12 hours before inoculated in 12-well plates in LB medium and M9 medium whose carbon source is glucose. The figure shows the green fluorescent intensity of E. coli under light of 460-465 nm wavelength with illuminated under around 2200 lux light. | <b>Figure 6/7</b> shows the date of the quantitative experimental characterization from SDU_CHINA 2019. Cells were cultured in the LB medium for 12 hours before inoculated in 12-well plates in LB medium and M9 medium whose carbon source is glucose. The figure shows the green fluorescent intensity of E. coli under light of 460-465 nm wavelength with illuminated under around 2200 lux light. | ||
<div style="display:flex;justify-content:space-around">[[File:T-SDU_CHINA-designfig21.png|300px|thumb|center|<b>Figure 6:</b> The relationship between the green fluorescent intensity of E. coli under light of 460-465nm wavelength and Illumination duration in LB medium.]][[File:T-SDU CHINA-BBaT3.png|300px|thumb|center|<b>Figure 7:</b> The relationship between the green fluorescent intensity of E. coli under light of 460-465nm wavelength and Illumination duration in M9 medium whose carbon source is glucose.]]</div> | <div style="display:flex;justify-content:space-around">[[File:T-SDU_CHINA-designfig21.png|300px|thumb|center|<b>Figure 6:</b> The relationship between the green fluorescent intensity of E. coli under light of 460-465nm wavelength and Illumination duration in LB medium.]][[File:T-SDU CHINA-BBaT3.png|300px|thumb|center|<b>Figure 7:</b> The relationship between the green fluorescent intensity of E. coli under light of 460-465nm wavelength and Illumination duration in M9 medium whose carbon source is glucose.]]</div> | ||
Latest revision as of 17:50, 20 October 2019
Blue light sensor YF1 and fixJ under constitutive promoter Lacclq35
This part contains a blue-light sensor comprised of YF1 (BBa_K592004) and fixJ (BBa_K592005), which can be switched off by 470 nm light and is on in its absence. When expressed, it can achieve 15-fold repression of PfixK2 (BBa_K592006). A strong synthetic terminator is added to avoid the re-use of parts, which can lead to homologous recombination. It is part of the GRB system, see more information from RGB System
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 587
Illegal NgoMIV site found at 659
Illegal NgoMIV site found at 749
Illegal NgoMIV site found at 767
Illegal NgoMIV site found at 1259
Illegal NgoMIV site found at 1552
Illegal NgoMIV site found at 1646
Illegal AgeI site found at 301
Illegal AgeI site found at 1427 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 1316
Illegal BsaI.rc site found at 200
Characterization
Figure 1 shows the relationship between the wavelength of light exposed on liquid medium and the intensity of BFP, GFP and RFP E. coli expressed from left figure to right figure respectively. We got the data through flow cytometer and analyzed it to get the figure. The y-axis is the number of cells, and the x-axis is fluorescence intensity. And every color is E. coli that grows for 8 hours under the light of the corresponding wavelength. We can see E. coli has the highest blue fluorescence expression under blue light from the left graph. And We can also see E. coli has the highest green and red fluorescence expression under green light and right light from the middle and right graph respectively. So this figure proves that our system and our parts can work well.
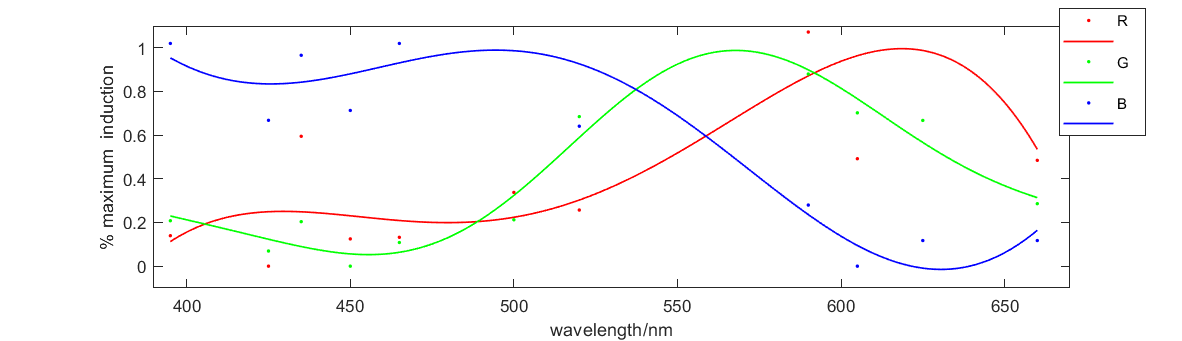
Figure 2 shows the relationship between fluorescence intensity and excitation wavelength. The x-axis is wavelength of 10h illumination. The solid medium gradually emerged and the y-axis is RGB figure of fluorescence in illuminated solid medium. This curve illustrates how our system responses to different excitation wavelength, which perfectly meets our expectation. So this figure proves that our system and our parts can work well.
Figure 3 shows colors we got from the solid medium exposed under light, in which E. coli producing fluorescent protein grows. When E .coli producing fluorescent protein are exposed under uniform light of single wavelength, the solid medium gradually emerged corresponding colors. And using color picker, we get many pure colors with predominant continuity.
We explored the relationship between fluorescent intensity and illumination intensity, which affects the shade of the color.
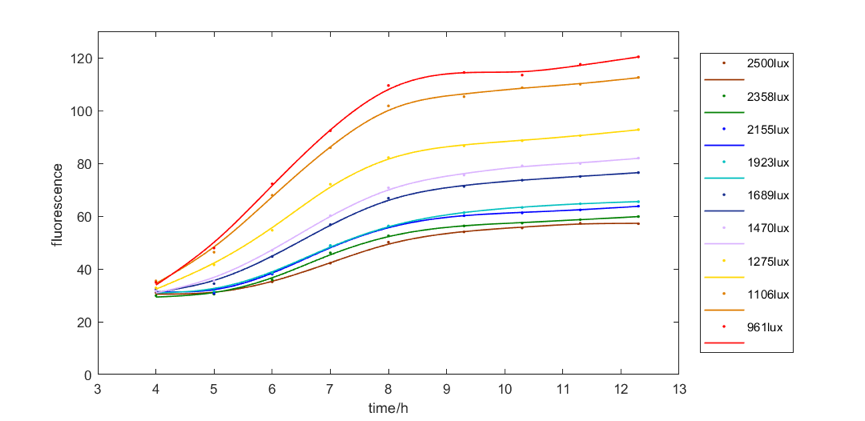
Figure 4 shows the red fluorescent intensity of E. coli under light of 620-630nm wavelength with different illumination intensity. We found when illuminated under around 961lux light, we can get the most red fluorescence. We also explore the relationship between green and blue fluorescent intensity of E. coli under light of 515-530nm wavelength and 460-470nm wavelength respectively and illumination intensity. The results are similar, that is, moderate intensity of light is most favorable for E. coli to express fluorescence.
Figure 5 shows results of gel electrophoresis of various parts after PCR with primer VF2\VR. The distance between primer binding sites and both ends of the parts are approximately 150 bp, thus rendering the product about 300 bp longer. The picture is edited to show a more compact photo.
Figure 6/7 shows the date of the quantitative experimental characterization from SDU_CHINA 2019. Cells were cultured in the LB medium for 12 hours before inoculated in 12-well plates in LB medium and M9 medium whose carbon source is glucose. The figure shows the green fluorescent intensity of E. coli under light of 460-465 nm wavelength with illuminated under around 2200 lux light.