Difference between revisions of "Part:BBa K2560002:Design"
(→Design Notes) |
(→Design Notes) |
||
| (One intermediate revision by the same user not shown) | |||
| Line 41: | Line 41: | ||
<html> | <html> | ||
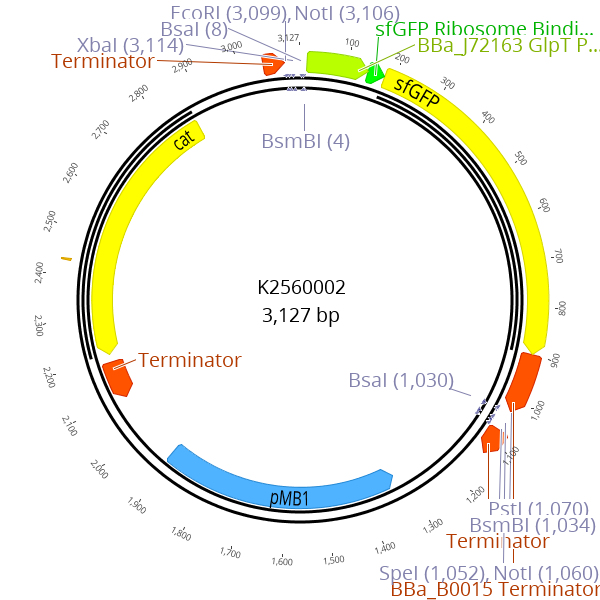
| − | <figcaption><b> Figure | + | <figcaption><b> Figure 1: K2560002 was designed as a novel part entry vector <br></b> A sfGFP dropout cassette is flanked by BsmBI recognition sites. During the cloning of LVL0 parts with BsmBI, this dropout cassette is replaced by the desired part </figcaption> |
</figure> | </figure> | ||
| Line 54: | Line 54: | ||
<a href="https://parts.igem.org/Part:BBa_BBa_ BBa_K2560002">BBa_K2560002</a> | <a href="https://parts.igem.org/Part:BBa_BBa_ BBa_K2560002">BBa_K2560002</a> | ||
, respectively. Like <a href="https://parts.igem.org/Part:BBa_P10500">BBa_P10500</a>, our two new dropout parts are located in a derivative of pSB1C3 which is extended with two BsaI recognition sites. The dropout parts are flanked by BsmBI recognition sites that are required for replacing the dropout by the desired LVL0 part. The resulting LVL0 parts are classified as PhytoBricks and are RFC10 and RFC25 compatible, assuming that the part itself does not contain prohibited recognition sites. The resulting plasmids can be used to clone and store all LVL0 parts. However, for resistance parts a different approach enables more convenient LVL1 cloning. In the LVL1 golden-gate-reaction, at least eight parts are combined in a single tube together with the required enzymes. All parts, except the resistance part, are stored in the previously described pSB1C3 derivate which confers a chloramphenicol resistance. Obviously, the plasmid that provides the new resistance cassette for the LVL1 plasmid (e.g. a Cas9 plasmid with kanamycin resistance), has to contain the same resistance cassette (kanamyicin in this example). Because cloning is never 100 % efficient, re-ligation of LVL0 plasmids is a common event, and in case of the LVL0 resistance part, results in false positive colonies which do not contain the desired LVL1 plasmid.<br> | , respectively. Like <a href="https://parts.igem.org/Part:BBa_P10500">BBa_P10500</a>, our two new dropout parts are located in a derivative of pSB1C3 which is extended with two BsaI recognition sites. The dropout parts are flanked by BsmBI recognition sites that are required for replacing the dropout by the desired LVL0 part. The resulting LVL0 parts are classified as PhytoBricks and are RFC10 and RFC25 compatible, assuming that the part itself does not contain prohibited recognition sites. The resulting plasmids can be used to clone and store all LVL0 parts. However, for resistance parts a different approach enables more convenient LVL1 cloning. In the LVL1 golden-gate-reaction, at least eight parts are combined in a single tube together with the required enzymes. All parts, except the resistance part, are stored in the previously described pSB1C3 derivate which confers a chloramphenicol resistance. Obviously, the plasmid that provides the new resistance cassette for the LVL1 plasmid (e.g. a Cas9 plasmid with kanamycin resistance), has to contain the same resistance cassette (kanamyicin in this example). Because cloning is never 100 % efficient, re-ligation of LVL0 plasmids is a common event, and in case of the LVL0 resistance part, results in false positive colonies which do not contain the desired LVL1 plasmid.<br> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
</p> | </p> | ||
</html> | </html> | ||
Latest revision as of 21:09, 17 October 2018
Phytobrick Entry Vector with GFP dropout
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 1
Illegal BsaI.rc site found at 1035
Illegal SapI.rc site found at 193
Design Notes
All LVL0 parts have to be stored in plasmids to allow for amplification and long term storage. To create new LVL0 parts, a PCR product or annealed oligos are cloned into a part entry vector. This vector harbours the resistance and ori that are required for selection and propagation. Furthermore, part entry vectors can be designed in a way that they contain a dropout. This dropout can be a transcription unit for a marker that generates a visible output. The first golden-gate-based toolbox MoClo (Weber et al. 2011.) used a LacZ alpha transcription unit which can be used for blue white screening in many E. coli cloning strains. This concept was also adapted by iGEMs PhytoBrick system. During the cloning of LVL0 parts, this dropout is replaced by the desired part. When the cloning reaction is transformed into a suitable E. coli strain and the cells are plated on agar plates with supplemented IPTG and X-Gal. Colonies transformed with the religated entry plasmid appear blue while white colonies most probably contain the correctly assembled plasmid. The LVL0 part entry vector in iGEMs PhytoBrick system (BBa_P10500) has been designed as described and can be used for blue white screening.
We appreciate the approach of using part entry plasmids with dropouts but, for two reasons, we think that LacZ is not an optimal reporter. First, blue white screening requires the two expensive chemicals IPTG and X-Gal which have to be added to the agar plates. Second, blue white screening is restricted to E.coli strains with an incomplete lac operon that is complemented by the LacZ alpha fragment that is expressed from the plasmid (Langley et al. 1975.) . Consequently blue white screening is not compatible with a V. natriegens wild type strain (Link zu Improvement Page).
A sfGFP dropout cassette is flanked by BsmBI recognition sites. During the cloning of LVL0 parts with BsmBI, this dropout cassette is replaced by the desired part
We overcame both drawbacks by replacing the LacZ alpha dropout of BBa_P10500 by a RFP and sfGFP dropout and thus created the two parts
BBa_K2560001
and
BBa_K2560002
, respectively. Like BBa_P10500, our two new dropout parts are located in a derivative of pSB1C3 which is extended with two BsaI recognition sites. The dropout parts are flanked by BsmBI recognition sites that are required for replacing the dropout by the desired LVL0 part. The resulting LVL0 parts are classified as PhytoBricks and are RFC10 and RFC25 compatible, assuming that the part itself does not contain prohibited recognition sites. The resulting plasmids can be used to clone and store all LVL0 parts. However, for resistance parts a different approach enables more convenient LVL1 cloning. In the LVL1 golden-gate-reaction, at least eight parts are combined in a single tube together with the required enzymes. All parts, except the resistance part, are stored in the previously described pSB1C3 derivate which confers a chloramphenicol resistance. Obviously, the plasmid that provides the new resistance cassette for the LVL1 plasmid (e.g. a Cas9 plasmid with kanamycin resistance), has to contain the same resistance cassette (kanamyicin in this example). Because cloning is never 100 % efficient, re-ligation of LVL0 plasmids is a common event, and in case of the LVL0 resistance part, results in false positive colonies which do not contain the desired LVL1 plasmid.
Source
The Phytobrick Acceptor Vector BBa_P10500 was used as a Template. LacZ cassette was replaced by sfGFP cassette which was PCR amplified from pYTK001 (Lee et al. 2015) and cloned into the vector with Gibson Assembly.
References
Lee M. E., DeLoache W. C., Cervantes B., Dieber J. E. (2015) A Highly Characterized Yeast Toolkit for Modular, Multipart Assembly, ACS Synth. Biol., 4(9):975-986