Difference between revisions of "PBAD SPL"
(→DTU pBAD SPL) |
(→The library) |
||
| (24 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | =Description= | |
| + | This is a page containing a synthetic promoter library (SPL) of pBAD promoters made by [http://2013.igem.org/Team:DTU-Denmark 2013 DTU iGEM team]. The library can be used to make and arabinose inducible system with very low leakiness, or tune the the promoter strength of the system when it's turned on. | ||
| − | + | A tight inducible system can be a very useful tool to express lethal or growth inhibiting proteins in ''E.coli''. It can also be necessary with a non leaky expression system if you would like to visualize the expression of GFP SF in ''E.coli'' (see [http://2013.igem.org/Team:DTU-Denmark/HelloWorld link]). | |
| + | |||
| + | |||
| + | |||
| + | =The library= | ||
| + | Here is a complete list of the sequences and their relative leakiness and strength: | ||
| − | |||
{|class="sortable" style="border:3px solid black; width: 70%" align="center" cellspacing="0" | {|class="sortable" style="border:3px solid black; width: 70%" align="center" cellspacing="0" | ||
! style="background:#AAAAAA; color:black" |Identifier | ! style="background:#AAAAAA; color:black" |Identifier | ||
! style="background:#AAAAAA; color:black" |Sequence<sup>a</sup> | ! style="background:#AAAAAA; color:black" |Sequence<sup>a</sup> | ||
! style="background:#AAAAAA; color:black" |Induced Strength | ! style="background:#AAAAAA; color:black" |Induced Strength | ||
| − | ! colspan=" | + | ! style="background:#AAAAAA; color:black" |Leakiness |
| + | ! colspan="3" style="background:#AAAAAA; color:black" |Inducibility<sup>b</sup> | ||
|- | |- | ||
| − | |align="center"|Col. | + | |align="center"|Col. 02 |
| − | |<font face="Courier" size="+1">CTGACGACGCCCCTCTCCGCCCCTTAAAATGTCCA</font> | + | |<font face="Courier" size="+1" class=">CTGACGACGCCCCTCTCCGCCCCTTAAAATGTCCA</font> |
|align="center"|14.2 | |align="center"|14.2 | ||
|align="center"|0.203 | |align="center"|0.203 | ||
| + | |align="center"|69.9 | ||
|- | |- | ||
| − | |align="center"|Col. | + | |align="center"|Col. 03 |
|<font face="Courier" size="+1">CTGACGTAATCATACCGCCGAAAGTATTATCATTA</font> | |<font face="Courier" size="+1">CTGACGTAATCATACCGCCGAAAGTATTATCATTA</font> | ||
|align="center"|16.6 | |align="center"|16.6 | ||
|align="center"|0.426 | |align="center"|0.426 | ||
| + | |align="center"|39 | ||
|- | |- | ||
| − | |align="center"|Col. | + | |align="center"|Col. 04 |
|<font face="Courier" size="+1">CTGACGCGACAACATTGCGTCCTATAAAATGCCGA</font> | |<font face="Courier" size="+1">CTGACGCGACAACATTGCGTCCTATAAAATGCCGA</font> | ||
|align="center"|14.2 | |align="center"|14.2 | ||
|align="center"|0.345 | |align="center"|0.345 | ||
| + | |align="center"|41 | ||
|- | |- | ||
| − | |align="center"|Col. | + | |align="center"|Col. 05 |
|<font face="Courier" size="+1">CTGACGGCCCGCCCCCGATGCGCATAAAATACCAA</font> | |<font face="Courier" size="+1">CTGACGGCCCGCCCCCGATGCGCATAAAATACCAA</font> | ||
|align="center"|17.7 | |align="center"|17.7 | ||
|align="center"|0.424 | |align="center"|0.424 | ||
| + | |align="center"|41.6 | ||
|- | |- | ||
|align="center"|Col. 12 | |align="center"|Col. 12 | ||
| Line 35: | Line 45: | ||
|align="center"|13.6 | |align="center"|13.6 | ||
|align="center"|0.414 | |align="center"|0.414 | ||
| + | |align="center"|32.9 | ||
|- | |- | ||
|align="center"|Col. 10 | |align="center"|Col. 10 | ||
| Line 40: | Line 51: | ||
|align="center"|18 | |align="center"|18 | ||
|align="center"|0.462 | |align="center"|0.462 | ||
| + | |align="center"|38.8 | ||
|- | |- | ||
| − | |align="center"|Col. | + | |align="center"|Col. 09 |
|<font face="Courier" size="+1">CTGACGCCACCCCCCCCCCCGGCGTATAATTCCCA</font> | |<font face="Courier" size="+1">CTGACGCCACCCCCCCCCCCGGCGTATAATTCCCA</font> | ||
|align="center"|16.1 | |align="center"|16.1 | ||
|align="center"|0.530 | |align="center"|0.530 | ||
| + | |align="center"|30.4 | ||
|- | |- | ||
| − | |align="center"|Col. | + | |align="center"|Col. 08 |
|<font face="Courier" size="+1">CTGACGCCCTACCGCTCGCCCCCCTATTATACCCA</font> | |<font face="Courier" size="+1">CTGACGCCCTACCGCTCGCCCCCCTATTATACCCA</font> | ||
|align="center"|13.4 | |align="center"|13.4 | ||
|align="center"|0.211 | |align="center"|0.211 | ||
| + | |align="center"|63.4 | ||
|- | |- | ||
|align="center"|Col. 13 | |align="center"|Col. 13 | ||
| Line 55: | Line 69: | ||
|align="center"|10.9 | |align="center"|10.9 | ||
|align="center"|0.096 | |align="center"|0.096 | ||
| + | |align="center"|113.6 | ||
|- | |- | ||
|align="center"|Col. 15 | |align="center"|Col. 15 | ||
| Line 60: | Line 75: | ||
|align="center"|15.5 | |align="center"|15.5 | ||
|align="center"|0.235 | |align="center"|0.235 | ||
| + | |align="center"|65.8 | ||
|- | |- | ||
|align="center"|Col. 18 | |align="center"|Col. 18 | ||
| Line 65: | Line 81: | ||
|align="center"|19.8 | |align="center"|19.8 | ||
|align="center"|0.524 | |align="center"|0.524 | ||
| + | |align="center"|37.8 | ||
|- | |- | ||
|align="center"|Col. 19 | |align="center"|Col. 19 | ||
| Line 70: | Line 87: | ||
|align="center"|18.0 | |align="center"|18.0 | ||
|align="center"|0.315 | |align="center"|0.315 | ||
| + | |align="center"|57 | ||
|- | |- | ||
|align="center"|Col. 31 | |align="center"|Col. 31 | ||
| Line 75: | Line 93: | ||
|align="center"|3.8 | |align="center"|3.8 | ||
|align="center"|0.161 | |align="center"|0.161 | ||
| + | |align="center"|23.6 | ||
|- | |- | ||
|align="center"|Col. 29 | |align="center"|Col. 29 | ||
| Line 80: | Line 99: | ||
|align="center"|14.8 | |align="center"|14.8 | ||
|align="center"|0.301 | |align="center"|0.301 | ||
| + | |align="center"|49.2 | ||
|- | |- | ||
|align="center"|Col. 26 | |align="center"|Col. 26 | ||
| Line 85: | Line 105: | ||
|align="center"|13.8 | |align="center"|13.8 | ||
|align="center"|0.292 | |align="center"|0.292 | ||
| + | |align="center"|47.2 | ||
|- | |- | ||
|align="center"|Col. 22 | |align="center"|Col. 22 | ||
| Line 90: | Line 111: | ||
|align="center"|16.8 | |align="center"|16.8 | ||
|align="center"|0.466 | |align="center"|0.466 | ||
| + | |align="center"|36.1 | ||
|- | |- | ||
|align="center"|Col. 33 | |align="center"|Col. 33 | ||
| Line 95: | Line 117: | ||
|align="center"|14.4 | |align="center"|14.4 | ||
|align="center"|1.01 | |align="center"|1.01 | ||
| + | |align="center"|14.3 | ||
|- | |- | ||
|align="center"|Col. 34 | |align="center"|Col. 34 | ||
| Line 100: | Line 123: | ||
|align="center"|12.4 | |align="center"|12.4 | ||
|align="center"|0.187 | |align="center"|0.187 | ||
| + | |align="center"|65.9 | ||
|- | |- | ||
|align="center"|Col. 35 | |align="center"|Col. 35 | ||
| Line 105: | Line 129: | ||
|align="center"|18.4 | |align="center"|18.4 | ||
|align="center"|0.89 | |align="center"|0.89 | ||
| + | |align="center"|20.7 | ||
|- | |- | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
|} | |} | ||
| + | |||
| + | <html> | ||
| + | <script> | ||
| + | function more_color(){ | ||
| + | var amino_string_containers = $("table font"); | ||
| + | for(var i=0; i < amino_string_containers.length; i++){ | ||
| + | var amino_string_container = amino_string_containers[i]; | ||
| + | var amino_string = amino_string_container.textContent; | ||
| + | amino_string_container.textContent = ""; | ||
| + | for(var j=0; j < amino_string.length; j++){ | ||
| + | var letter = amino_string[j]; | ||
| + | var color = "black"; | ||
| + | //if( letter == "C"){ | ||
| + | // color = "green"; | ||
| + | //} else if( letter == "A"){ | ||
| + | // color = "blue"; | ||
| + | //} else if( letter == "T"){ | ||
| + | // color = "orange"; | ||
| + | //} else if( letter == "G"){ | ||
| + | // color = "red"; | ||
| + | //} | ||
| + | //$(amino_string_container).append("<font color=" + color + ">" + letter + "</font>"); | ||
| + | if( letter == "C"){ | ||
| + | color = "rgb(26,204,26)"; | ||
| + | } else if( letter == "A"){ | ||
| + | color = "rgb(128,179,230)"; | ||
| + | } else if( letter == "T"){ | ||
| + | color = "rgb(230,153,77)"; | ||
| + | } else if( letter == "G"){ | ||
| + | color = "rgb(230,128,128)"; | ||
| + | } | ||
| + | $(amino_string_container).append('<font style="background-color: ' + color + '">' + letter + '</font>'); | ||
| + | } | ||
| + | } | ||
| + | } | ||
| + | more_color(); | ||
| + | </script> | ||
| + | </html> | ||
| + | |||
| + | <sup>a</sup> The sequence of each pBAD promoter characterized. To use this simply produce a primer with the promoter sequence you wish in a tail. Replace the old promoter in your system e.g. [[Part:BBa_K808000]] with the new and see the chance. For more information go to ([http://2013.igem.org/Team:DTU-Denmark/pBAD_SPL link]). | ||
| + | |||
| + | <sup>b</sup> Inducibility = Induced Strength divided by leakiness. | ||
| + | |||
| + | =Characterization= | ||
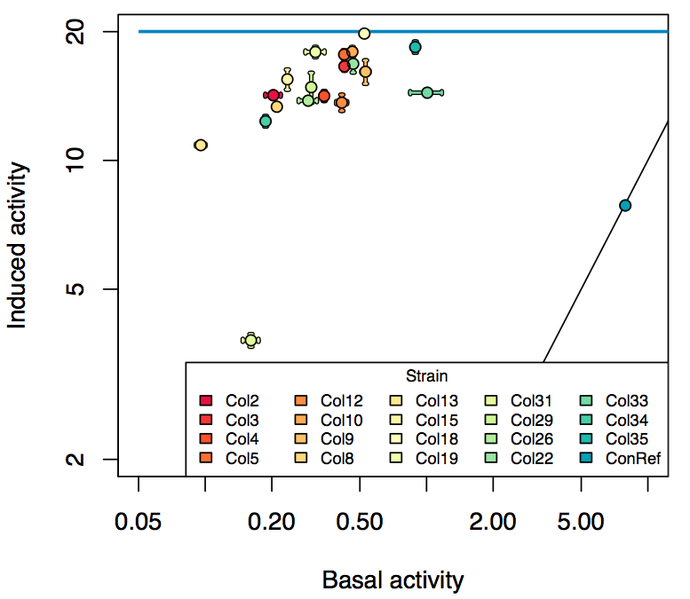
| + | We did a characterization by running growth experiments in parallel on a BioLector ([http://2013.igem.org/Team:DTU-Denmark/pBAD_SPL see more]). Below diagram sums up our results: | ||
| + | [[File:690px-Induced vs basal.png|600px|center|Diagram over the promoter strength]] | ||
| + | |||
| + | =Conclusion= | ||
| + | The pBAD SPL contain promoters to the arabinose inducible system that will enable low leakiness but still maintain a reasonable promoter strength when turned on. This verifies that the SPL standard ([http://dspace.mit.edu/handle/1721.1/60080 RFC 63]) also can be used to produce promoters for inducible systems. | ||
Latest revision as of 22:06, 4 October 2013
Description
This is a page containing a synthetic promoter library (SPL) of pBAD promoters made by [http://2013.igem.org/Team:DTU-Denmark 2013 DTU iGEM team]. The library can be used to make and arabinose inducible system with very low leakiness, or tune the the promoter strength of the system when it's turned on.
A tight inducible system can be a very useful tool to express lethal or growth inhibiting proteins in E.coli. It can also be necessary with a non leaky expression system if you would like to visualize the expression of GFP SF in E.coli (see [http://2013.igem.org/Team:DTU-Denmark/HelloWorld link]).
The library
Here is a complete list of the sequences and their relative leakiness and strength:
| Identifier | Sequencea | Induced Strength | Leakiness | Inducibilityb | ||
|---|---|---|---|---|---|---|
| Col. 02 | CTGACGACGCCCCTCTCCGCCCCTTAAAATGTCCA | 14.2 | 0.203 | 69.9 | ||
| Col. 03 | CTGACGTAATCATACCGCCGAAAGTATTATCATTA | 16.6 | 0.426 | 39 | ||
| Col. 04 | CTGACGCGACAACATTGCGTCCTATAAAATGCCGA | 14.2 | 0.345 | 41 | ||
| Col. 05 | CTGACGGCCCGCCCCCGATGCGCATAAAATACCAA | 17.7 | 0.424 | 41.6 | ||
| Col. 12 | CTGACGCGTATCGCGAGCGGGCGTTATTATACGCA | 13.6 | 0.414 | 32.9 | ||
| Col. 10 | CTGACGCCCCGAATCAGTAGTATTTATTATCTAGA | 18 | 0.462 | 38.8 | ||
| Col. 09 | CTGACGCCACCCCCCCCCCCGGCGTATAATTCCCA | 16.1 | 0.530 | 30.4 | ||
| Col. 08 | CTGACGCCCTACCGCTCGCCCCCCTATTATACCCA | 13.4 | 0.211 | 63.4 | ||
| Col. 13 | CTGACGCCCCCCCCGTCCACCCCCTAATATCCCGA | 10.9 | 0.096 | 113.6 | ||
| Col. 15 | CTGACGTGCGCCTGCCGTCCAAAGTAATATCCTTA | 15.5 | 0.235 | 65.8 | ||
| Col. 18 | CTGACGCCCCGAATCAGTAGTATTTATTATCTAGA | 19.8 | 0.524 | 37.8 | ||
| Col. 19 | CTGACGCTCGCACCGGCGACCCGATAATATCCATA | 18.0 | 0.315 | 57 | ||
| Col. 31 | CTGACGCCCCCCCTCCCCCGACCTAAAATATCCAG | 3.8 | 0.161 | 23.6 | ||
| Col. 29 | CTGACGCCGCTGCACCCGTCCCCCTATTATAGCAA | 14.8 | 0.301 | 49.2 | ||
| Col. 26 | CTGACGCCGATCCTATCTCCCTATTAAAATTTTTA | 13.8 | 0.292 | 47.2 | ||
| Col. 22 | CTGACGCCCCGAATCAGTAGTATTTATTATCTAGA | 16.8 | 0.466 | 36.1 | ||
| Col. 33 | CTGACGCCAGCTTCCTACTTCAAATATAATCCGTA | 14.4 | 1.01 | 14.3 | ||
| Col. 34 | CTGACGTCCCTCTTCCGCGCCCCCTAAAATACCCA | 12.4 | 0.187 | 65.9 | ||
| Col. 35 | CTGACGCCCAACCCGACACAGCGATATAATAATGA | 18.4 | 0.89 | 20.7 | ||
a The sequence of each pBAD promoter characterized. To use this simply produce a primer with the promoter sequence you wish in a tail. Replace the old promoter in your system e.g. Part:BBa_K808000 with the new and see the chance. For more information go to ([http://2013.igem.org/Team:DTU-Denmark/pBAD_SPL link]).
b Inducibility = Induced Strength divided by leakiness.
Characterization
We did a characterization by running growth experiments in parallel on a BioLector ([http://2013.igem.org/Team:DTU-Denmark/pBAD_SPL see more]). Below diagram sums up our results:
Conclusion
The pBAD SPL contain promoters to the arabinose inducible system that will enable low leakiness but still maintain a reasonable promoter strength when turned on. This verifies that the SPL standard ([http://dspace.mit.edu/handle/1721.1/60080 RFC 63]) also can be used to produce promoters for inducible systems.