Difference between revisions of "Part:BBa K875009"
(→SZU-China 2021:LL37 Inflammation suppression effect) |
|||
| (50 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
| − | |||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K875009 short</partinfo> | <partinfo>BBa_K875009 short</partinfo> | ||
| − | + | The LL 37 cathelicidin is a human antimicrobial peptide. | |
| + | |||
| + | The exact mechanism by which AMPs kill microorganisms is still under debate. | ||
| + | |||
| + | The eukaryotic toxin LL 37 was obtained by synthesis with the canonical prefix and suffix RFC10 compatible and it was optimized for E. coli expression. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| Line 13: | Line 20: | ||
<partinfo>BBa_K875009 SequenceAndFeatures</partinfo> | <partinfo>BBa_K875009 SequenceAndFeatures</partinfo> | ||
| + | ==Tec-Chihuahua 2019: Effect of LL 37 in <i>E. coli</i> growth== | ||
| + | <p align="justify"> | ||
| + | Team [https://2019.igem.org/Team:Tec-Chihuahua Tec-Chihuahua] worked on the characterization of BBa_K875009. We realized that the few characterizations of LL 37 were all qualitative. This is why our team undertook the task of characterizing this part in order to report a quantitative absorbance study. | ||
| + | LL 37 has antimicrobial activity against both Gram-positive and Gram-negative bacteria. <i>Escherichia coli</i> is susceptible to the effects of this peptide, and since it's one of the most common chassis for recombinant protein production, the effects that LL 37 may have on the growth of <i>E. coli</i> when used for its expression were evaluated. | ||
| + | <br> | ||
| + | Expression of toxic proteins may adversely affect the viability of the chassis. In order to test <i>E. coli</i> as a viable host for LL 37 production, <i>E. coli</i> BL21 (DE3) cells were transformed with part BBa_K2959000 which is a composite capable of expressing LL 37 under the regulation of the T7 promoter. Three flasks with 30 mL of LB broth were inoculated with 3 mL of an overnight culture of transformed cells. Another flask with 30 mL of LB broth was inoculated with 3 mL of untransformed cells to use as a control. The four flasks were incubated at 37°C and 225 rpm until OD<sub>600</sub> reached 0.8. The flasks with transformed cells were treated with different concentrations of IPTG (0.1, 0.5, and 1 mM) to induce protein production. Then, optical density was measured every hour for five hours to measure growth. Results were recorded in the following table and chart. | ||
| + | </p> | ||
| + | <br> | ||
| + | <center> <b>Table 1. </b>OD<sub>600</sub> of transformed and untransformed <i>E. coli</i> BL21 (DE3) cultures treated with different concentrations of IPTG.</center> | ||
| + | <table class="lab-table" style="margin-left: auto; margin-right: auto;"> | ||
| + | <tr> | ||
| + | <th style="background-color: #80BA6B"> </th> | ||
| + | <th style="background-color: #80BA6B">0 h</th> | ||
| + | <th style="background-color: #80BA6B">1 h</th> | ||
| + | <th style="background-color: #80BA6B">2 h</th> | ||
| + | <th style="background-color: #80BA6B">3 h</th> | ||
| + | <th style="background-color: #80BA6B">4 h</th> | ||
| + | <th style="background-color: #80BA6B">5 h</th> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td> Untransformed BL21 (DE3)</td> | ||
| + | <td> 0.859</td> | ||
| + | <td> 1.238</td> | ||
| + | <td> 1.511</td> | ||
| + | <td> 1.636</td> | ||
| + | <td> 1.709</td> | ||
| + | <td> 1.752</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td> Transformed BL21 (DE3), 0.1 mM IPTG </td> | ||
| + | <td> 0.784</td> | ||
| + | <td> 1.238</td> | ||
| + | <td> 1.362</td> | ||
| + | <td> 1.451</td> | ||
| + | <td> 1.549</td> | ||
| + | <td> 1.6</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td> Transformed BL21 (DE3), 0.5 mM IPTG </td> | ||
| + | <td> 0.80</td> | ||
| + | <td> 1.218</td> | ||
| + | <td> 1.346</td> | ||
| + | <td> 1.418</td> | ||
| + | <td> 1.51</td> | ||
| + | <td> 1.573</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td> Transformed BL21 (DE3), 1.0 mM IPTG </td> | ||
| + | <td> 0.81</td> | ||
| + | <td> 1.197</td> | ||
| + | <td> 1.297</td> | ||
| + | <td> 1.351</td> | ||
| + | <td> 1.427</td> | ||
| + | <td> 1.481</td> | ||
| + | </tr> | ||
| + | </table> | ||
| − | < | + | |
| − | ===Functional Parameters== | + | <p align="justify"> |
| + | The results obtained show a distinct difference in absorbance, which correlates to culture growth, between untransformed and transformed <i>E. coli</i>. After 5 hours of incubation, untransformed BL21 (DE3) (blue line) indicated an OD<sub>600</sub> of 1.752. Previous data, compared to transformed BL21 (DE3) with LL37 (yellow line) with the highest induction condition and time (1mM of IPTG and 5 hours), shows an OD<sub>600</sub> of 1.481 which denotes a reduction in growth of approximately <b>15.5% </b>. | ||
| + | Untransformed cells grew better indicating that <b>LL 37 production has a negative effect on bacterial growth</b>. Concentration of IPTG and absorbance showed an inversely proportional relationship. Higher concentrations of IPTG must induce a larger production of LL 37 and, due to its toxicity towards its host, it may have caused this partial inhibition. Thus, expression of LL 37 reduces bacterial growth; however, no significant reduction in total growth was perceived. In conclusion, inhibition is not significant enough to consider expression of LL 37 in <i>E. coli</i> BL21 (DE3) non-viable. | ||
| + | </p> | ||
| + | <br> | ||
| + | <center>https://2019.igem.org/wiki/images/5/50/T--Tec-Chihuahua--GRAFICA.png</center> | ||
| + | <center><b>Fig.1 </b> Absorbance curve measured at 600 nm of transformed and untransformed <i>E. coli</i> BL21 (DE3) cultures treated with different concentrations of IPTG.</center> | ||
| + | <br> | ||
| + | |||
| + | ===SZU-China 2021:LL37 Inflammation suppression effect=== | ||
| + | SZU-China team has added quantitative experimental characterization data to an existing Part from the Registry of Standard Biological Parts and documented the experimental characterization on the Part's Main Page on the Registry. | ||
| + | |||
| + | Due to the small size of the LL37 target protein and the problems encountered in protein purification, we failed to purify and collect the expressed target protein for subsequent experiments. After reviewing the literature, we found that LL37 has powerful anti-endotoxin properties and can regulate inflammatory response by inhibiting the release of pro-inflammatory cytokine TNF-α in LPS-stimulated human monocytes. This proves that our project design is scientific and feasible.[1] | ||
| + | |||
| + | [[File:T--SZU-China--POCll37.png|400px|center]] | ||
| + | Figure 1 A, THP-1 cells were stimulated with 10 or 100 ng/ml LPS in the presence of increasing concentrations of LL-37 (x-axis) for 4 h. B, PBMCs were stimulated with 100 ng/ml LPS in presence or absence of 20 ug/ml LL-37 for 4 h. The anti-endotoxin effect of LL-37 demonstrated in PBMC was statistically significant with a value of p 0.05 (**). | ||
| + | |||
| + | Reference | ||
| + | [1] Neeloffer Mookherjee K L B D. Human Host Defense Peptide LL-37 Inflammatory Response by the Endogenous Modulation of the TLR-Mediated[J]. The Journal of Immunology, 2021. | ||
| + | |||
| + | ==Functional Parameters: Austin_UTexas== | ||
| + | <html> | ||
| + | <body> | ||
<partinfo>BBa_K875009 parameters</partinfo> | <partinfo>BBa_K875009 parameters</partinfo> | ||
| − | < | + | <h3><center>Burden Imposed by this Part:</center></h3> |
| + | <figure> | ||
| + | <div class = "center"> | ||
| + | <center><img src = "https://static.igem.org/mediawiki/parts/f/fa/T--Austin_Utexas--no_burden_icon.png" style = "width:160px;height:120px"></center> | ||
| + | </div> | ||
| + | <figcaption><center><b>Burden Value: 0.8 ± 2.2% </b></center></figcaption> | ||
| + | </figure> | ||
| + | <p> Burden is the percent reduction in the growth rate of <i>E. coli</i> cells transformed with a plasmid containing this BioBrick (± values are 95% confidence limits). This BioBrick did not exhibit a burden that was significantly greater than zero (i.e., it appears to have little to no impact on growth). Therefore, users can depend on this part to remain stable for many bacterial cell divisions and in large culture volumes. Refer to any one of the | ||
| + | <a href="https://parts.igem.org/Part:BBa_K3174002">BBa_K3174002</a> - <a href="https://parts.igem.org/Part:BBa_K3174007">BBa_K3174007</a> pages for more information on the methods, an explanation of the sources of burden, and other conclusions from a large-scale measurement project conducted by the <a href="http://2019.igem.org/Team:Austin_UTexas">2019 Austin_UTexas team</a>.</p> | ||
| + | <p>This functional parameter was added by the <a href="https://2020.igem.org/Team:Austin_UTexas/Contribution">2020 Austin_UTexas team.</a></p> | ||
| + | </body> | ||
| + | </html> | ||
Latest revision as of 16:57, 21 October 2021
LL 37 - Cathelicidin
The LL 37 cathelicidin is a human antimicrobial peptide.
The exact mechanism by which AMPs kill microorganisms is still under debate.
The eukaryotic toxin LL 37 was obtained by synthesis with the canonical prefix and suffix RFC10 compatible and it was optimized for E. coli expression.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
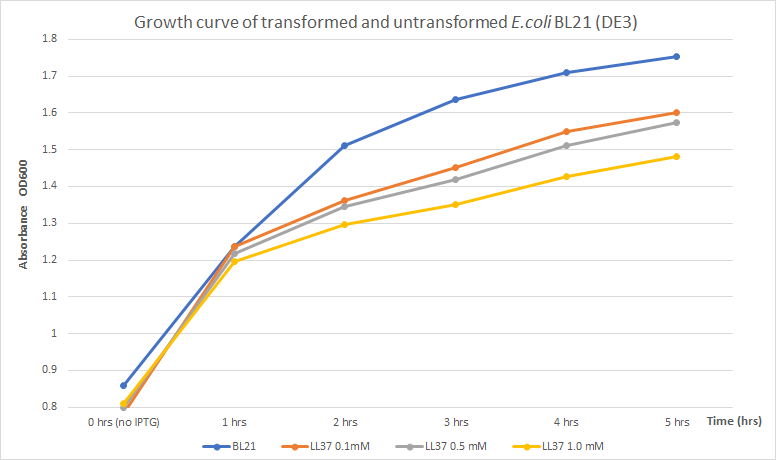
Tec-Chihuahua 2019: Effect of LL 37 in E. coli growth
Team Tec-Chihuahua worked on the characterization of BBa_K875009. We realized that the few characterizations of LL 37 were all qualitative. This is why our team undertook the task of characterizing this part in order to report a quantitative absorbance study.
LL 37 has antimicrobial activity against both Gram-positive and Gram-negative bacteria. Escherichia coli is susceptible to the effects of this peptide, and since it's one of the most common chassis for recombinant protein production, the effects that LL 37 may have on the growth of E. coli when used for its expression were evaluated.
Expression of toxic proteins may adversely affect the viability of the chassis. In order to test E. coli as a viable host for LL 37 production, E. coli BL21 (DE3) cells were transformed with part BBa_K2959000 which is a composite capable of expressing LL 37 under the regulation of the T7 promoter. Three flasks with 30 mL of LB broth were inoculated with 3 mL of an overnight culture of transformed cells. Another flask with 30 mL of LB broth was inoculated with 3 mL of untransformed cells to use as a control. The four flasks were incubated at 37°C and 225 rpm until OD600 reached 0.8. The flasks with transformed cells were treated with different concentrations of IPTG (0.1, 0.5, and 1 mM) to induce protein production. Then, optical density was measured every hour for five hours to measure growth. Results were recorded in the following table and chart.
| 0 h | 1 h | 2 h | 3 h | 4 h | 5 h | |
|---|---|---|---|---|---|---|
| Untransformed BL21 (DE3) | 0.859 | 1.238 | 1.511 | 1.636 | 1.709 | 1.752 |
| Transformed BL21 (DE3), 0.1 mM IPTG | 0.784 | 1.238 | 1.362 | 1.451 | 1.549 | 1.6 |
| Transformed BL21 (DE3), 0.5 mM IPTG | 0.80 | 1.218 | 1.346 | 1.418 | 1.51 | 1.573 |
| Transformed BL21 (DE3), 1.0 mM IPTG | 0.81 | 1.197 | 1.297 | 1.351 | 1.427 | 1.481 |
The results obtained show a distinct difference in absorbance, which correlates to culture growth, between untransformed and transformed E. coli. After 5 hours of incubation, untransformed BL21 (DE3) (blue line) indicated an OD600 of 1.752. Previous data, compared to transformed BL21 (DE3) with LL37 (yellow line) with the highest induction condition and time (1mM of IPTG and 5 hours), shows an OD600 of 1.481 which denotes a reduction in growth of approximately 15.5% . Untransformed cells grew better indicating that LL 37 production has a negative effect on bacterial growth. Concentration of IPTG and absorbance showed an inversely proportional relationship. Higher concentrations of IPTG must induce a larger production of LL 37 and, due to its toxicity towards its host, it may have caused this partial inhibition. Thus, expression of LL 37 reduces bacterial growth; however, no significant reduction in total growth was perceived. In conclusion, inhibition is not significant enough to consider expression of LL 37 in E. coli BL21 (DE3) non-viable.
SZU-China 2021:LL37 Inflammation suppression effect
SZU-China team has added quantitative experimental characterization data to an existing Part from the Registry of Standard Biological Parts and documented the experimental characterization on the Part's Main Page on the Registry.
Due to the small size of the LL37 target protein and the problems encountered in protein purification, we failed to purify and collect the expressed target protein for subsequent experiments. After reviewing the literature, we found that LL37 has powerful anti-endotoxin properties and can regulate inflammatory response by inhibiting the release of pro-inflammatory cytokine TNF-α in LPS-stimulated human monocytes. This proves that our project design is scientific and feasible.[1]
Figure 1 A, THP-1 cells were stimulated with 10 or 100 ng/ml LPS in the presence of increasing concentrations of LL-37 (x-axis) for 4 h. B, PBMCs were stimulated with 100 ng/ml LPS in presence or absence of 20 ug/ml LL-37 for 4 h. The anti-endotoxin effect of LL-37 demonstrated in PBMC was statistically significant with a value of p 0.05 (**).
Reference [1] Neeloffer Mookherjee K L B D. Human Host Defense Peptide LL-37 Inflammatory Response by the Endogenous Modulation of the TLR-Mediated[J]. The Journal of Immunology, 2021.
Functional Parameters: Austin_UTexas
Burden Imposed by this Part:

Burden is the percent reduction in the growth rate of E. coli cells transformed with a plasmid containing this BioBrick (± values are 95% confidence limits). This BioBrick did not exhibit a burden that was significantly greater than zero (i.e., it appears to have little to no impact on growth). Therefore, users can depend on this part to remain stable for many bacterial cell divisions and in large culture volumes. Refer to any one of the BBa_K3174002 - BBa_K3174007 pages for more information on the methods, an explanation of the sources of burden, and other conclusions from a large-scale measurement project conducted by the 2019 Austin_UTexas team.
This functional parameter was added by the 2020 Austin_UTexas team.

