Difference between revisions of "Part:BBa K729001"
Sednanalien (Talk | contribs) (→Results) |
ProfessorLi (Talk | contribs) |
||
| (8 intermediate revisions by 3 users not shown) | |||
| Line 20: | Line 20: | ||
The protein IrrE originates from ''Deinococcus radiodurans'', where it confers resistance to radiation. When transformed into ''E. Coli'' however, it protected against salt, oxidative and thermal shock. IrrE appears to function as a global regulator of stress factor genes. So far it has been demonstrated to upregulate transcription of recA and pprA – genes which encode Recombinase A and Radiation Inducible Protein. With respect to salt tolerance, IrrE upregulates the production of several stress responsive proteins, protein kinases, metabolic proteins, and detoxification proteins. It also downregulates glycerol degradation. With this global regulatory effect, ''E. Coli'' becomes more salt tolerant. | The protein IrrE originates from ''Deinococcus radiodurans'', where it confers resistance to radiation. When transformed into ''E. Coli'' however, it protected against salt, oxidative and thermal shock. IrrE appears to function as a global regulator of stress factor genes. So far it has been demonstrated to upregulate transcription of recA and pprA – genes which encode Recombinase A and Radiation Inducible Protein. With respect to salt tolerance, IrrE upregulates the production of several stress responsive proteins, protein kinases, metabolic proteins, and detoxification proteins. It also downregulates glycerol degradation. With this global regulatory effect, ''E. Coli'' becomes more salt tolerant. | ||
| + | |||
| + | In order to characterise this BioBrick, we ligated it with the Constitutive Promoter <partinfo>BBa_J23119</partinfo>, and Ribosome Binding Site <partinfo>BBa_B0034</partinfo>. This produced the final construct, <partinfo>BBa_K729005</partinfo>. | ||
===Characterisation=== | ===Characterisation=== | ||
| Line 27: | Line 29: | ||
===Results=== | ===Results=== | ||
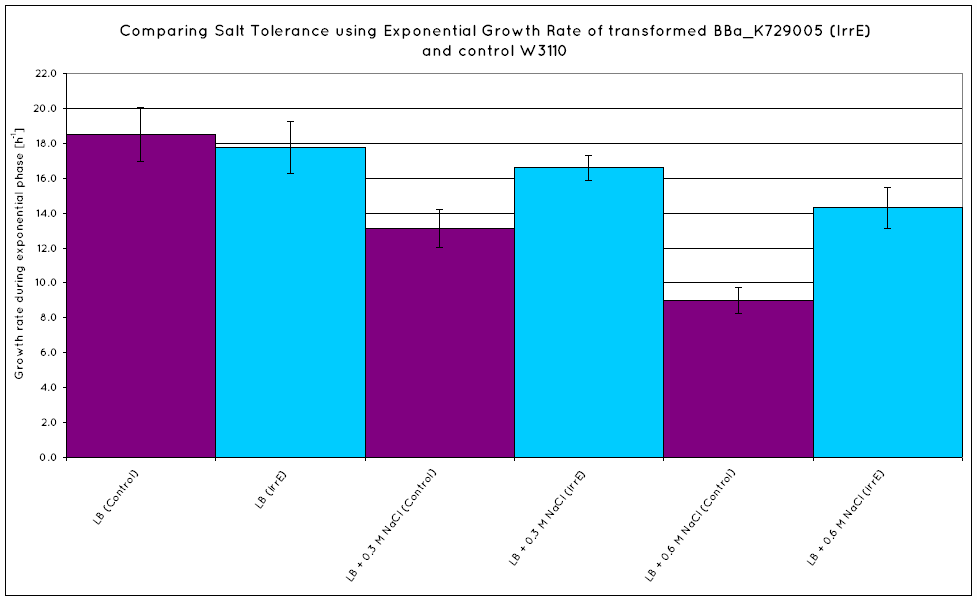
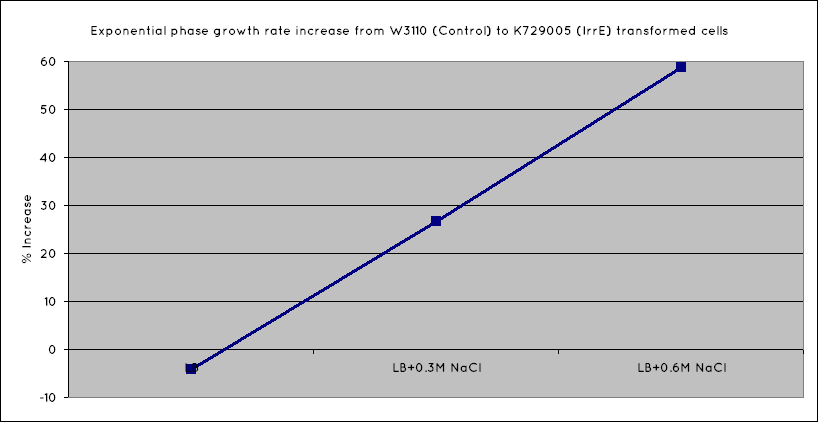
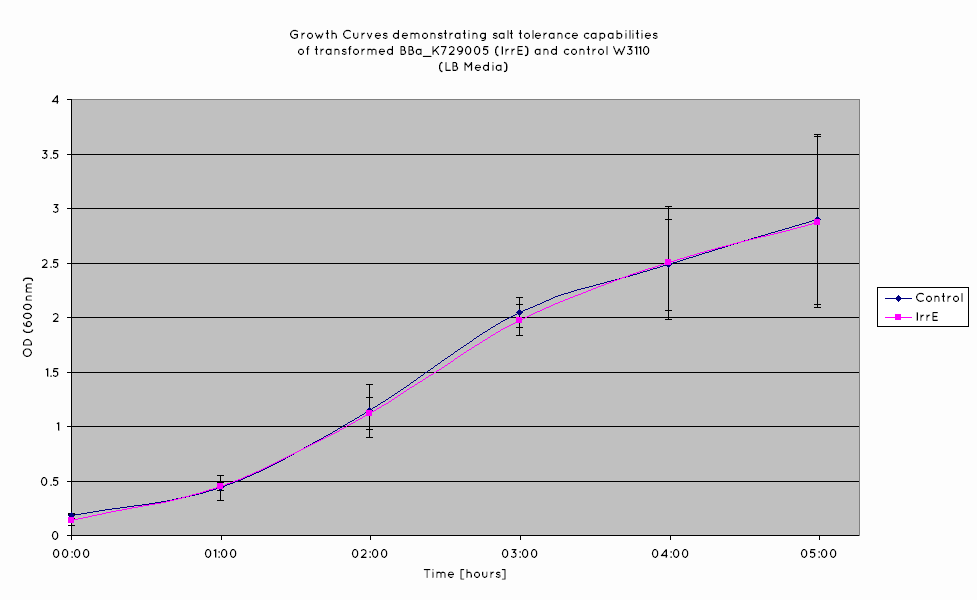
From our results, we observed that E. Coli transformed with the IrrE gene exhibit greater salt tolerance than their non-transformed counterparts. This is revealed in the greater OD the cells grow to in high salt media, as well as their increased growth rate. Our results are consistent with the results obtained in the original paper (Pan ''et al.'' 2009), suggesting that our cells are indeed expressing the IrrE global regulator. | From our results, we observed that E. Coli transformed with the IrrE gene exhibit greater salt tolerance than their non-transformed counterparts. This is revealed in the greater OD the cells grow to in high salt media, as well as their increased growth rate. Our results are consistent with the results obtained in the original paper (Pan ''et al.'' 2009), suggesting that our cells are indeed expressing the IrrE global regulator. | ||
| + | |||
| + | [[Image:UniversityCollegeLondon_IrrE_Growth_Rate.png|470px]] | ||
| + | [[Image:UniversityCollegeLondon_IrrE_Growth_Rate_%_Increase.png|470px]] | ||
| + | [[Image:UniversityCollegeLondon_IrrE_Growth_Curves_LB.png|470px]] | ||
| + | [[Image:UniversityCollegeLondon_IrrE_Growth_Curves_LB%2B0.3.png|470px]] | ||
| + | [[Image:UniversityCollegeLondon_IrrE_Growth_Curves_LB%2B0.6.png|470px|centre]] | ||
===Modelling=== | ===Modelling=== | ||
| Line 33: | Line 41: | ||
We have determined that IrrE is an ideal global regulatory gene to be utilised in conferring salt tolerance on our cells. Transforming our cells with the IrrE gene, we have successfully increase the growth rate of E. Coli in high salinity conditions. | We have determined that IrrE is an ideal global regulatory gene to be utilised in conferring salt tolerance on our cells. Transforming our cells with the IrrE gene, we have successfully increase the growth rate of E. Coli in high salinity conditions. | ||
| + | ===Improvement=== | ||
| + | |||
| + | <html> | ||
| + | Team <a href="http://2017.igem.org/Team:ITB_Indonesia">ITB_Indonesia</a> made sequence improvement on this part through <a href="https://parts.igem.org/Part:BBa_K2378007">BBa_K2378007</a>. | ||
| + | </html> | ||
===References=== | ===References=== | ||
"IrrE, a global regulator of extreme radiation resistance in Deinococcus radiodurans, enhances salt tolerance in Escherichia coli and Brassica napus.(Pan, J., Wang, J., Zhou, Z.(2009)PloS one, 4(2), e4422.) | "IrrE, a global regulator of extreme radiation resistance in Deinococcus radiodurans, enhances salt tolerance in Escherichia coli and Brassica napus.(Pan, J., Wang, J., Zhou, Z.(2009)PloS one, 4(2), e4422.) | ||
| + | |||
| + | ==Added by LZU-HS-China-E== | ||
| + | |||
| + | ===Growth under Salt Stress=== | ||
| + | |||
| + | After we have successfully constructed our engineered bacteria, we need to compare its functions. At this stage, we need different groups to make a comparison so that we can more intuitively show what changes the added genes have made to our engineered bacteria. | ||
| + | Now we will compare three different groups of strains. One group is the control group. That is, DH5alpha, which has not been changed at all, has not let us insert any genes. That is, the strain that has not been genetically modified is used as the control group. The second group added T7 promoter. The third group is the final product of our experiment, which adds the complete sequence, that is, the ire gene with the GroESL promoter to help its gene expression. However, in the end, we will put them together and observe their growth under different salinity. | ||
| + | |||
| + | When the concentration was 0%, the control group grew the fastest at the same time. Because the control group that adds nothing at this time has the least burden. Engineering bacteria with foreign genes such as T7 and GroESL will have more burdens and make their growth more difficult. | ||
| + | |||
| + | Without these, the burden will be much smaller when growing, and this is under the condition of a salinity of 0. However, the growth of these strains is not very good, because certain salinity is also indispensable for the growth of strains. In this case, the control group with a small burden will have better advantages. But GroESL and ire with salt tolerance genes will grow better than T7. This is when the salt concentration is 0. | ||
| + | |||
| + | [[Image:T--LZU-HS-China-E--China-E1.jpg | thumb | center | 500px |Figure 1 ]] | ||
| + | |||
| + | If the concentration of salt increases to 1%, the overall growth rate will exceed the situation when the salinity is 0%. Because an appropriate salt concentration can increase the growth rate of bacteria. But at this time, GroESL is still faster than T7. But the control group is still the fastest because there is no high salinity to affect the growth of bacteria, so its additional genes still have no effect. These salinities are acceptable to bacteria themselves, and more genes still add a burden to them. | ||
| + | |||
| + | [[Image:T--LZU-HS-China-E--China-E2.jpg | thumb | center | 500px |Figure 2 ]] | ||
| + | |||
| + | When the concentration of salt reaches 5%, the overall growth rate will be affected and drop a lot. After that, the role of GroESL and global regulators appeared. It exceeded the control group and T7 with the highest growth efficiency. But T7 is still at the bottom. In the end, when the salt content reaches 7%, the growth efficiency of all three groups will be almost zero because of the too high salt concentration, which means that it is almost impossible for them to grow at this time because it has become very difficult for them to survive under that condition. | ||
| + | |||
| + | [[Image:T--LZU-HS-China-E--China-E3.jpg | thumb | center | 500px |Figure 3 ]] | ||
| + | |||
| + | ===Growth of colonies=== | ||
| + | |||
| + | We verified the number of colonies formed by different strains under different salt concentrations. Z represents the insertion of InP, EC20, gro promoter and IrrE global regulators to increase salt tolerance and heavy metal adsorption capacity, gro represents the insertion of only gro promoter and IrrE global regulators to improve salt tolerance, and C represents the control group without any insertion. From the following chart, it can be seen that the colonies formed by three different strains in saline gradually decrease with the increase of salt concentration. | ||
| + | The strain represented by Z has always formed the fewest colonies among the three strains, because it has not only inserted the gro promoter and the ire fragment for improving salinity tolerance, but also inserted the EC20 and InP fragments for adsorbing heavy metals, which makes it too burdensome to grow as fast as other strains. When the concentration of saline reaches 20%, the strain represented by Z basically stops growing. | ||
| + | |||
| + | [[Image:T--LZU-HS-China-E--China-E4.jpg | thumb | center | 500px |Figure 4 ]] | ||
| + | |||
| + | The strain represented by gro has always been the fastest growing strain because it only inserts the gene fragments of the gro promoter and the ire global regulator, which does not make its growth burden heavier, and the insertion of the ire global regulator improves its salt tolerance so that it can grow better in saline. The strain represented by gro basically stopped growing when the saline concentration reached 25%. | ||
Latest revision as of 06:23, 11 October 2022
irrE from Deinococcus radiodurans
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Description
The protein IrrE originates from Deinococcus radiodurans, where it confers resistance to radiation. When transformed into E. Coli however, it protected against salt, oxidative and thermal shock. IrrE appears to function as a global regulator of stress factor genes. So far it has been demonstrated to upregulate transcription of recA and pprA – genes which encode Recombinase A and Radiation Inducible Protein. With respect to salt tolerance, IrrE upregulates the production of several stress responsive proteins, protein kinases, metabolic proteins, and detoxification proteins. It also downregulates glycerol degradation. With this global regulatory effect, E. Coli becomes more salt tolerant.
In order to characterise this BioBrick, we ligated it with the Constitutive Promoter BBa_J23119, and Ribosome Binding Site BBa_B0034. This produced the final construct, BBa_K729005.
Characterisation
IrrE is deduced to globally regulate gene expression in E. Coli, and to date, no specific assay allows for the convenient determination of IrrE expression. As such, the expression of IrrE will be determined indirectly by determining the increased salt tolerance of our cells. In order to confirm this, we will be conducting growth curve experiments. This will allow us to determine if our cell grow to a higher density in high salinity when compared to the untransformed cells.
Results
From our results, we observed that E. Coli transformed with the IrrE gene exhibit greater salt tolerance than their non-transformed counterparts. This is revealed in the greater OD the cells grow to in high salt media, as well as their increased growth rate. Our results are consistent with the results obtained in the original paper (Pan et al. 2009), suggesting that our cells are indeed expressing the IrrE global regulator.
Modelling
Conclusion
We have determined that IrrE is an ideal global regulatory gene to be utilised in conferring salt tolerance on our cells. Transforming our cells with the IrrE gene, we have successfully increase the growth rate of E. Coli in high salinity conditions.
Improvement
Team ITB_Indonesia made sequence improvement on this part through BBa_K2378007.
References
"IrrE, a global regulator of extreme radiation resistance in Deinococcus radiodurans, enhances salt tolerance in Escherichia coli and Brassica napus.(Pan, J., Wang, J., Zhou, Z.(2009)PloS one, 4(2), e4422.)
Added by LZU-HS-China-E
Growth under Salt Stress
After we have successfully constructed our engineered bacteria, we need to compare its functions. At this stage, we need different groups to make a comparison so that we can more intuitively show what changes the added genes have made to our engineered bacteria. Now we will compare three different groups of strains. One group is the control group. That is, DH5alpha, which has not been changed at all, has not let us insert any genes. That is, the strain that has not been genetically modified is used as the control group. The second group added T7 promoter. The third group is the final product of our experiment, which adds the complete sequence, that is, the ire gene with the GroESL promoter to help its gene expression. However, in the end, we will put them together and observe their growth under different salinity.
When the concentration was 0%, the control group grew the fastest at the same time. Because the control group that adds nothing at this time has the least burden. Engineering bacteria with foreign genes such as T7 and GroESL will have more burdens and make their growth more difficult.
Without these, the burden will be much smaller when growing, and this is under the condition of a salinity of 0. However, the growth of these strains is not very good, because certain salinity is also indispensable for the growth of strains. In this case, the control group with a small burden will have better advantages. But GroESL and ire with salt tolerance genes will grow better than T7. This is when the salt concentration is 0.
If the concentration of salt increases to 1%, the overall growth rate will exceed the situation when the salinity is 0%. Because an appropriate salt concentration can increase the growth rate of bacteria. But at this time, GroESL is still faster than T7. But the control group is still the fastest because there is no high salinity to affect the growth of bacteria, so its additional genes still have no effect. These salinities are acceptable to bacteria themselves, and more genes still add a burden to them.
When the concentration of salt reaches 5%, the overall growth rate will be affected and drop a lot. After that, the role of GroESL and global regulators appeared. It exceeded the control group and T7 with the highest growth efficiency. But T7 is still at the bottom. In the end, when the salt content reaches 7%, the growth efficiency of all three groups will be almost zero because of the too high salt concentration, which means that it is almost impossible for them to grow at this time because it has become very difficult for them to survive under that condition.
Growth of colonies
We verified the number of colonies formed by different strains under different salt concentrations. Z represents the insertion of InP, EC20, gro promoter and IrrE global regulators to increase salt tolerance and heavy metal adsorption capacity, gro represents the insertion of only gro promoter and IrrE global regulators to improve salt tolerance, and C represents the control group without any insertion. From the following chart, it can be seen that the colonies formed by three different strains in saline gradually decrease with the increase of salt concentration. The strain represented by Z has always formed the fewest colonies among the three strains, because it has not only inserted the gro promoter and the ire fragment for improving salinity tolerance, but also inserted the EC20 and InP fragments for adsorbing heavy metals, which makes it too burdensome to grow as fast as other strains. When the concentration of saline reaches 20%, the strain represented by Z basically stops growing.
The strain represented by gro has always been the fastest growing strain because it only inserts the gene fragments of the gro promoter and the ire global regulator, which does not make its growth burden heavier, and the insertion of the ire global regulator improves its salt tolerance so that it can grow better in saline. The strain represented by gro basically stopped growing when the saline concentration reached 25%.