Difference between revisions of "Part:BBa K525304"
JSchwarzhans (Talk | contribs) (→Important parameters) |
|||
| (9 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K525304 short</partinfo> | <partinfo>BBa_K525304 short</partinfo> | ||
| + | |||
| + | [[Image:Bielefeld-Germany2011-S-Layer-Geometrien.jpg|300px|thumb|right]] | ||
Fusion protein of S-layer SgsE and mCherry RFP | Fusion protein of S-layer SgsE and mCherry RFP | ||
| Line 30: | Line 32: | ||
|expression of T7 polymerase + IPTG or lactose | |expression of T7 polymerase + IPTG or lactose | ||
|- | |- | ||
| − | |Specific growth rate | + | |Specific growth rate (un-/induced) |
| − | |0.128 h<sup>-1</sup> / 0.094 h<sup>-1</sup> | + | |0.128 h<sup>-1</sup> / 0.094 h<sup>-1</sup> |
|- | |- | ||
| − | |Doubling time | + | |Doubling time (un-/induced) |
| − | |5.41 h / 7.39 h | + | |5.41 h / 7.39 h |
|- | |- | ||
|rowspan="3"|Purification | |rowspan="3"|Purification | ||
| Line 65: | Line 67: | ||
===Expression in ''E. coli''=== | ===Expression in ''E. coli''=== | ||
| − | The SgsE ( | + | The SgsE gene under the control of a T7 / lac promoter (<partinfo>K525303</partinfo>) was fused to mCherry ([https://parts.igem.org/Part:BBa_J18932 BBa_J18932]) using Freiburg BioBrick assembly for characterization experiments. |
| + | |||
| + | The SgsE|mCherry fusion protein was overexpressed in ''E. coli'' KRX after induction of T7 polymerase by supplementation of 0.1 % L-rhamnose and 1 mM IPTG using the autinduction protocol by Promega. | ||
| + | |||
| + | |||
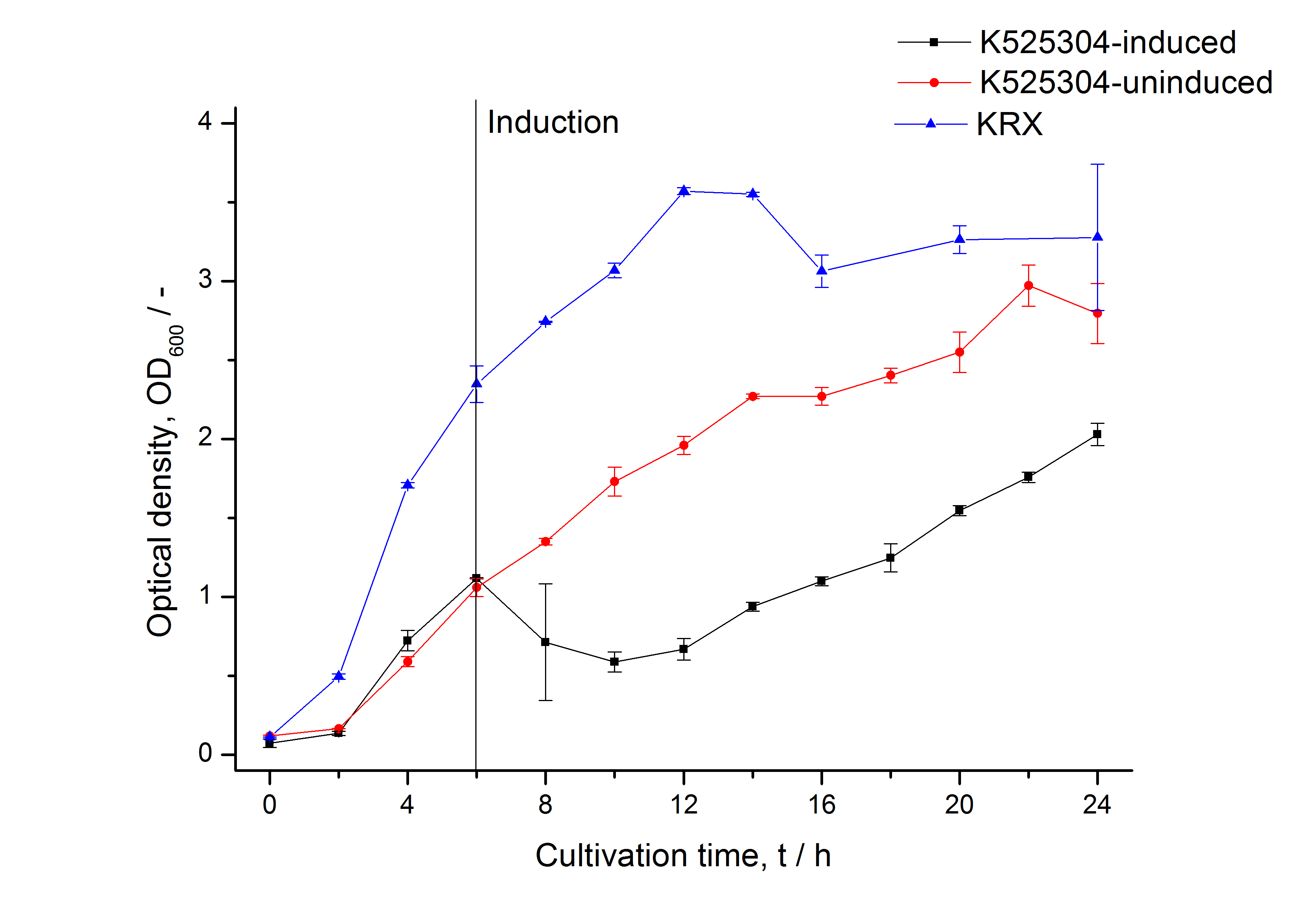
| + | [[Image:Bielefeld_2011_304_Growthcurve1.png|600px|center|thumb| '''Figure 1: Growth curve of ''E. coli'' KRX expressing the fusion protein of SgsE and mCherry with and without induction, cultivated at 37 °C in autoinduction medium with and without inductor, respectively. A curve depicting KRX wildtype is shown for comparsion. After induction at approximately 6 h the OD<sub>600</sub> of the induced <partinfo>K525304</partinfo> visibly drops when compared to the uninduced culture. Both cultures grow significantly slower than KRX wildtype.''']] | ||
| + | |||
| + | [[Image:Bielefeld_2011_304_RFU_OD.png|600px|center|thumb| '''Figure 2: RFU to OD<sub>600</sub> ratio of ''E. coli'' KRX expressing the fusion protein of SgsE and mCherry RFP with and without induction. A curve depicting KRX wildtype is shown for comparsion. After induction at approximately 6 h the RFU to OD<sub>600</sub> ratio starts to rise in the induced culture. Compared to the uninduced culture the ratio is roughly two times higher. The KRX wildtype shows no variation in the RFU to OD<sub>600</sub> ratio.''']] | ||
| + | |||
| + | |||
| + | ===Methods=== | ||
| + | |||
| + | '''Expression of S-layer genes in ''E. coli'' ''' | ||
| + | * Chassis: Promega's [http://www.promega.com/products/cloning-and-dna-markers/cloning-tools-and-competent-cells/bacterial-strains-and-competent-cells/single-step-_krx_-competent-cells/ ''E. coli'' KRX] | ||
| + | |||
| + | * Medium: LB medium supplemented with 20 mg L<sup>-1</sup> chloramphenicol | ||
| + | ** For autoinduction: Cultivations in LB-medium were supplemented with 0.1 % L-rhamnose and 1 mM IPTG as inducer and 0.05 % glucose | ||
| + | |||
| + | |||
| + | '''Measuring of [https://parts.igem.org/Part:BBa_E1010 mRFP]''' | ||
| + | * Take at least 500 µL sample for each measurement (200 µL is needed for one measurement) so you can perform a repeat determination | ||
| + | * Freeze biological samples at -80 °C for storage, keep cell-free at 4 °C in the dark | ||
| + | * To measure the samples thaw at room temperature and fill 200 µL of each sample in one well of a black, flat bottom 96 well microtiter plate (perform at least a repeat determination) | ||
| + | * Measure the fluorescence in a platereader (we used a [http://www.tecan.com/platform/apps/product/index.asp?MenuID=1812&ID=1916&Menu=1&Item=21.2.10.1 Tecan Infinite® M200 platereader]) with following settings: | ||
| + | ** 20 sec orbital shaking (1 mm amplitude with a frequency of 87.6 rpm) | ||
| + | ** Measurement mode: Top | ||
| + | ** Excitation: 584 nm | ||
| + | ** Emission: 620 nm | ||
| + | ** Number of reads: 25 | ||
| + | ** Manual gain: 100 | ||
| + | ** Integration time: 20 µs | ||
| − | |||
| − | + | ===References=== | |
| + | Kainz B, Steiner K, Möller M, Pum D, Schäffer C, Sleytr UB, Toca-Herrera JL (2010) Absorption, Steady-State Fluorescence, Fluorescence Lifetime, and 2D Self-Assembly Properties of Engineered Fluorescent S-Layer Fusion Proteins of ''Geobacillus stearothermophilus'' NRS 2004/3a, [http://pubs.acs.org/doi/abs/10.1021/bm901071b ''Biomacromolecules'' 11(1):207-214]. | ||
| − | [ | + | Sleytr UB, Huber C, Ilk N, Pum D, Schuster B, Egelseer EM (2007) S-layers as a tool kit for nanobiotechnological applications, [http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full ''FEMS Microbiol Lett'' 267(2):131-144]. |
Latest revision as of 03:15, 22 September 2011
Fusion Protein of S-Layer SgsE and mCherry RFP
Fusion protein of S-layer SgsE and mCherry RFP
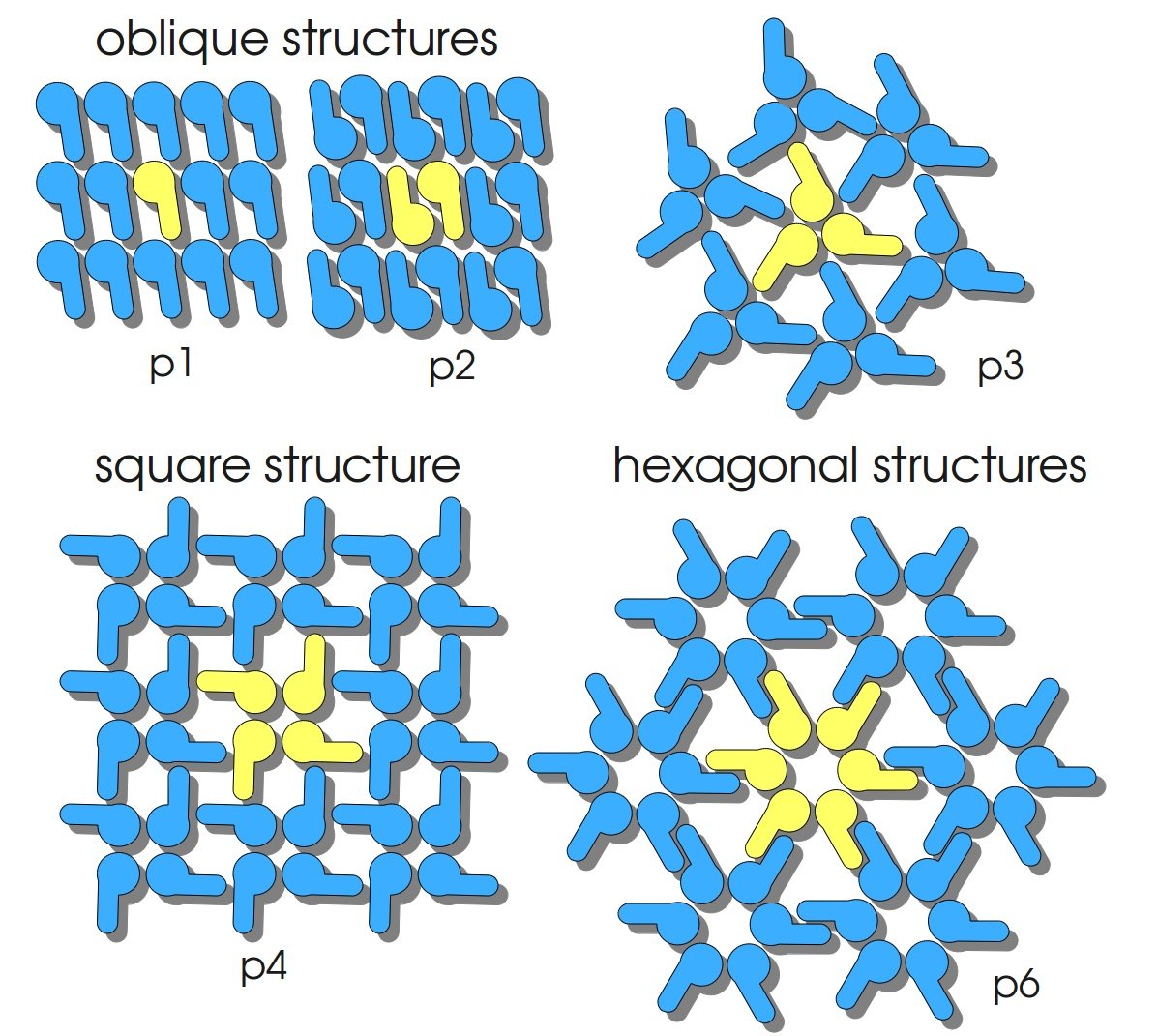
S-layers (crystalline bacterial surface layer) are crystal-like layers consisting of multiple protein monomers and can be found in various (archae-)bacteria. They constitute the outermost part of the cell wall. Especially their ability for self-assembly into distinct geometries is of scientific interest. At phase boundaries, in solutions and on a variety of surfaces they form different lattice structures. The geometry and arrangement is determined by the C-terminal self assembly-domain, which is specific for each S-layer protein. The most common lattice geometries are oblique, square and hexagonal. By modifying the characteristics of the S-layer through combination with functional groups and protein domains as well as their defined position and orientation to eachother (determined by the S-layer geometry) it is possible to realize various practical applications ([http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full Sleytr et al., 2007]).
Usage and Biology
S-layer proteins can be used as scaffold for nanobiotechnological applications and devices by e.g. fusing the S-layer's self-assembly domain to other functional protein domains. It is possible to coat surfaces and liposomes with S-layers. A big advantage of S-layers: after expressing in E. coli and purification, the nanobiotechnological system is cell-free. This enhances the biological security of a device.
This fluorescent S-layer fusion protein is used to characterize purification methods and the S-layer's ability to self-assemble on surfaces. It is also possible to use the characteristic of mCherry as a pH indicator ([http://pubs.acs.org/doi/abs/10.1021/bm901071b Kainz et al., 2010]).
Important parameters
| Experiment | Characteristic | Result |
|---|---|---|
| Expression (E. coli) | Localisation | Inclusion body |
| Compatibility | E. coli KRX and BL21(DE3) | |
| Induction of expression | expression of T7 polymerase + IPTG or lactose | |
| Specific growth rate (un-/induced) | 0.128 h-1 / 0.094 h-1 | |
| Doubling time (un-/induced) | 5.41 h / 7.39 h | |
| Purification | Molecular weight | 109.9 kDa |
| Theoretical pI | 5.70 | |
| Excitation / emission | 587 / 610 nm | |
| Immobilization behaviour | Immobilization time | 4 h |
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 167
Illegal BglII site found at 1022 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 76
Illegal AgeI site found at 3112 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 1657
Expression in E. coli
The SgsE gene under the control of a T7 / lac promoter (BBa_K525303) was fused to mCherry (BBa_J18932) using Freiburg BioBrick assembly for characterization experiments.
The SgsE|mCherry fusion protein was overexpressed in E. coli KRX after induction of T7 polymerase by supplementation of 0.1 % L-rhamnose and 1 mM IPTG using the autinduction protocol by Promega.

Methods
Expression of S-layer genes in E. coli
- Chassis: Promega's [http://www.promega.com/products/cloning-and-dna-markers/cloning-tools-and-competent-cells/bacterial-strains-and-competent-cells/single-step-_krx_-competent-cells/ E. coli KRX]
- Medium: LB medium supplemented with 20 mg L-1 chloramphenicol
- For autoinduction: Cultivations in LB-medium were supplemented with 0.1 % L-rhamnose and 1 mM IPTG as inducer and 0.05 % glucose
Measuring of mRFP
- Take at least 500 µL sample for each measurement (200 µL is needed for one measurement) so you can perform a repeat determination
- Freeze biological samples at -80 °C for storage, keep cell-free at 4 °C in the dark
- To measure the samples thaw at room temperature and fill 200 µL of each sample in one well of a black, flat bottom 96 well microtiter plate (perform at least a repeat determination)
- Measure the fluorescence in a platereader (we used a [http://www.tecan.com/platform/apps/product/index.asp?MenuID=1812&ID=1916&Menu=1&Item=21.2.10.1 Tecan Infinite® M200 platereader]) with following settings:
- 20 sec orbital shaking (1 mm amplitude with a frequency of 87.6 rpm)
- Measurement mode: Top
- Excitation: 584 nm
- Emission: 620 nm
- Number of reads: 25
- Manual gain: 100
- Integration time: 20 µs
References
Kainz B, Steiner K, Möller M, Pum D, Schäffer C, Sleytr UB, Toca-Herrera JL (2010) Absorption, Steady-State Fluorescence, Fluorescence Lifetime, and 2D Self-Assembly Properties of Engineered Fluorescent S-Layer Fusion Proteins of Geobacillus stearothermophilus NRS 2004/3a, [http://pubs.acs.org/doi/abs/10.1021/bm901071b Biomacromolecules 11(1):207-214].
Sleytr UB, Huber C, Ilk N, Pum D, Schuster B, Egelseer EM (2007) S-layers as a tool kit for nanobiotechnological applications, [http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full FEMS Microbiol Lett 267(2):131-144].