Difference between revisions of "Part:BBa R0010"
| (101 intermediate revisions by 13 users not shown) | |||
| Line 12: | Line 12: | ||
* LacI is coded by <bbpart>BBa_C0010</bbpart> | * LacI is coded by <bbpart>BBa_C0010</bbpart> | ||
| + | Intrinsic noise value: 0.0707 (compare with R0011: 0.0040; R0051: 0.0869). See [http://2015.igem.org/Team:William_and_Mary William_and_Mary iGEM 2015] | ||
| + | |||
| + | |||
| + | |||
| + | >Internal Priming Screening Characterization of BBa_R0010: Has 3 possible internal priming site between this BioBrick part and the VR primer. | ||
| + | |||
| + | The 2018 Hawaii iGEM team evaluated the 40 most frequently used BioBricks and ran them through an internal priming screening process that we developed using the BLAST program tool. Out of the 40 BioBricks we evaluated, 10 of them showed possible internal priming of either the VF2 or VR primers and sometime even both. The data set has a range of sequence lengths from as small as 12 bases to as large as 1,210 bases. We experienced the issue of possible internal priming during the sequence verification process of our own BBa_K2574001 BioBrick and in the cloning process to express the part as a fusion protein. BBa_K2574001 is a composite part containing a VLP forming Gag protein sequence attached to a frequently used RFP part (BBa_E1010). We conducted a PCR amplification of the Gag-RFP insert using the VF2 and VR primers on the ligation product (pSB1C3 ligated to the Gag + RFP). This amplicon would serve as template for another PCR where we would add the NcoI and BamHI restriction enzyme sites through new primers for ligation into pET14b and subsequent induced expression. Despite gel confirming a rather large, approximately 2.1 kb insert band, our sequencing results with the VR primer and BamHI RFP reverse primer gave mixed results. Both should have displayed the end of the RFP, but the VR primer revealed the end of the Gag. Analysis of the VR primer on the Gag-RFP sequence revealed several sites where the VR primer could have annealed with ~9 - 12 bp of complementarity. Internal priming of forward and reverse primers can be detrimental to an iGEM project because you can never be sure if the desired construct was correctly inserted into the BioBrick plasmid without a successful sequence verification. | ||
| + | |||
| + | For the BioBrick part BBa_R0010, the first location of the internal priming site is on the 121-113 base number of the BioBrick and on the 12-20 base number of the VR primer. The second location of the internal priming site is on the 11-17 base number of the BioBrick and on the 4-10 base number of the VR primer. The third location of the internal priming site is on the 84-90 base number of the BioBrick and on the 14-20 base number of the VR primer. | ||
| Line 18: | Line 27: | ||
This is a direct copy of bases 0365739 through 0365540 of the E. coli K-12 MG1655 genome, Genbank NC_000913 in reverse complement form. It is the natural promoter for the LacZYA operon. | This is a direct copy of bases 0365739 through 0365540 of the E. coli K-12 MG1655 genome, Genbank NC_000913 in reverse complement form. It is the natural promoter for the LacZYA operon. | ||
It includes the tail end of the LacI gene coding region, but no promoter region for that partial gene. | It includes the tail end of the LacI gene coding region, but no promoter region for that partial gene. | ||
| − | |||
| − | |||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
<partinfo>BBa_R0010 SequenceAndFeatures</partinfo> | <partinfo>BBa_R0010 SequenceAndFeatures</partinfo> | ||
| + | ==Usage in <i>Chromobacterium Violaceum</i>== | ||
| − | <!-- | + | [http://2016.igem.org/Team:Tec-Monterrey Team Tec-Monterrey 2016] tested the Promoter BBa_R0010 in <i>C. Violaceum</i>. We were unable to confirm whether or not <i>C. Violaceum</i> had the lac operon, BLAST analysis of its genome suggested that it did not have this regulating system; our characterization demonstrates that the lac promoter can be used as a constitutive promoter in this chassis. There was no significant difference in the Fluorescence of the group of <i>C. Violaceum</i> with IPTG or without it, but in <i>E. Coli</i> it showed normal induction. Therefore, we can confirm that this promoter is constitutive in <i>C. Violaceum</i>. https://static.igem.org/mediawiki/parts/4/4b/T--Tec-Monterrey--violaceumgrafiicauno.jpg https://static.igem.org/mediawiki/parts/7/76/T--Tec-Monterrey--violaceumgrafiicados.jpg |
| − | ===Functional Parameters== | + | |
| + | |||
| + | ==Characterization for usage in genetically recoded organisms and expanded genetic code== | ||
| + | |||
| + | [https://2019.igem.org/Team:Aboa Team Aboa 2019]used this biobrick in production optimization ofcell strain C321.ΔA.exp using expanded genetic code in expression for modified proteins. This biobrick is completely functional and usable also for production of proteins modified with unnatural amino acids. | ||
| + | |||
| + | For the measurements we used Cytation5 in overnight incubation and shaking at 37 degrees celsius. Fluorescence was measured every 30 minutes at preset bandwith using GFP and mCherry programs. Relative fluorescence is calculated as fluorescence at the start of induction with IPTG, pAzF and arabinose divided by the end of incubation the next morning. | ||
| + | |||
| + | https://2019.igem.org/wiki/images/3/38/T--Aboa--GFP-TAG-RFP--chart.png | ||
| + | |||
| + | |||
| + | |||
| + | The relative change of RFP fluorescence divided by the change of GFP fluorescence after induction with variable amounts of p-azido-l-phenylalanine, IPTG and arabinose. Relative change of fluorescence is used to normalize signals that are present before induction. The figure shows that IPTG concentrations does not have an effect on the expression of RFP as the promoter is leaky and the arabinose and pAzF are much more critical for producing proteins with unnatural amino acid modifications. High relative expression when little or no pAzF or arabinose is present is most likely a sign of misincorporation of amino acids in avoidance of ribosome stalling. | ||
| + | |||
| + | https://2019.igem.org/wiki/images/b/b4/T--Aboa--GFP-TAG-RFP--chartIPTG.png | ||
| + | |||
| + | Average eelative change of fluorescence from each concentration of IPTG. | ||
| + | |||
| + | |||
| + | ==Usage of the <i>lac</i> promoter to express fluorescent markers in two different species== | ||
| + | |||
| + | [https://2019.igem.org/Team:Hong_Kong_HKU Hong_Kong_HKU] tested this promoter (BBa_R0010) in two different species, Escherichia coli and Salmonella Typhimurium to test whether this is a constitutive promoter in S. Typhi. | ||
| + | |||
| + | E. coli and S. Typhimurium belong to the same order, Enterobacteriaceae. Lactose fermentation ability is used to differentiate them. S. Typhimurium does not have the ability to ferment lactose like E. coli does. In this characterisation experiment, this lac promoter is transformed to S. Typhimurium and E. coli as a control to see the difference in the expression level of the downstream red fluorescent markers. | ||
| + | |||
| + | https://2019.igem.org/wiki/images/c/c8/T--Hong_Kong_HKU--lacoperon.png | ||
| + | |||
| + | As the data suggests, this promoter is a constitutive promoter in S. Typhimurium as there is no difference in signals with or without IPTG. As expected, under IPTG induction, there is a 3-fold increase in RFP signals compared to those without IPTG. | ||
| + | |||
| + | ==Tec-Chihuahua 2019: Usage of lacI promoter to express RFP into <i>E.coli </i>SHuffle T7 Express== | ||
| + | <p align="justify"> | ||
| + | Team [https://2019.igem.org/Team:Tec-Chihuahua Tec-Chihuahua]evaluated the functionality of this promoter by testing it in <i>E. coli</i> expression strain SHuffle T7 Express®️. SHuffle was chosen as chassis for having a cytoplasmic environment that favors the production of disulfide bonds. A designed was made for the composite parts under the regulation of part BBa_R0010, a lacI regulated promoter inducible by IPTG. However,characterization of promoters in the iGEM Parts Registry for their use in SHuffle strains is nonexistent. | ||
| + | |||
| + | <br> | ||
| + | To evaluate protein production by part BBa_R0010, part BBa_J04450 was transformed, an RFP coding device under the regulation of this promoter, into <i>E. coli</i> SHuffle T7 Express®️. Transformed cells were grown on LB agar with chloramphenicol, for BBa_J04450 was located in the pSB1C3 backbone. | ||
| + | <br> | ||
| + | RFP expression was induced at different temperature conditions and IPTG concentration to determine the ideal conditions for the proper functionality of the promoter. Protein expression was analysed through an SDS-PAGE. | ||
| + | <br> | ||
| + | The functionality of promoter BBa_R0010 in <i>E. coli</i> SHuffle T7 Express®️ was demonstrated as well as ideal conditions for protein production by characterizing part BBa_J04450, an RFP construct. <i>E. coli</i> SHuffle T7 Express®️ produces higher amounts of protein at 30°C, and concentrations of 0.2 and 0.4 mM IPTG are enough to induce expression when using this lacI regulated promoter. | ||
| + | </p> | ||
| + | <br> | ||
| + | <center> <b>Table 1. </b>Conditions of induction for characterization of production of RFP.</center> | ||
| + | <table class="lab-table" style="margin-left: auto; margin-right: auto;"> | ||
| + | <tr> | ||
| + | <th style="background-color: #80BA6B">Well</th> | ||
| + | <th style="background-color: #80BA6B">37°C</th> | ||
| + | <th style="background-color: #80BA6B">Well</th> | ||
| + | <th style="background-color: #80BA6B">30°C</th> | ||
| + | |||
| + | </tr> | ||
| + | <tr> | ||
| + | <td> Negative control</td> | ||
| + | <td> Untransformed control</td> | ||
| + | <td> Negative control</td> | ||
| + | <td> Untransformed control</td> | ||
| + | |||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><center> 1 </center></td> | ||
| + | <td> Uninduced control (without IPTG)</td> | ||
| + | <td><center> 4 </center> </td> | ||
| + | <td> Uninduced control (without IPTG)</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td> <center> 2 </center> </td> | ||
| + | <td>Transformed cells, 0.2 mM IPTG</td> | ||
| + | <td> <center> 5 </center></td> | ||
| + | <td>Transformed cells, 0.2 mM IPTG | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td> <center> 3 </center> </td> | ||
| + | <td>Transformed cells, 0.4 mM IPTG</td> | ||
| + | <td> <center> 6 </center></td> | ||
| + | <td>Transformed cells, 0.4 mM IPTG</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | <br> | ||
| + | <center>[[File:T--Tec-Chihuahua--RFPGEL.jpeg|500px]]</center> | ||
| + | <center><b>Figure 1.</b> SDS-PAGE (12%) of <i>E. coli </i>SHuffle T7 Express®️ transformed with BBa_J04450. (Negative control:<i>E.coli</i> Shuffle untransformed cells at 37°C, 1.RFP transformed cells without induction, 2.RFP transformed cells with 0.2mM of IPTG at 37°C, 3.RFP transformed cells with 0.4mM of IPTG at 37°C, Negative control: <i>E.coli</i> Shuffle untransformed cells at 30°C, 4.RFP transformed cells without induction, 5.RFP transformed cells with 0.2mM of IPTG at 30°C, 6.RFP transformed cells with 0.4mM of IPTG at 30°C) </center> | ||
| + | <br> | ||
| + | <p align="justify"> | ||
| + | Two prominent bands can be appreciated in the last two columns, corresponding to induction conditions of 0.2 and 0.4 mM IPTG at 30°C, close to the 25 kDa band of the Precision Plus ProteinTM Dual Xtra Prestained Protein Standards. It was concluded that they correspond to RFP, a 26 kDa protein. | ||
| + | <br> | ||
| + | |||
| + | When taking a closer look, the band corresponding to RFP can be seen in each column with exception of the untransformed cells controls (negative controls). The uninduced controls (column 1 for 37°C and column 4 for 30°C) without IPTG were expected not to show bands if the promoter lacked leakiness; nevertheless, a faint band can be seen for both controls. Bands corresponding to 0.2 and 0.4 mM IPTG for both temperatures (columns 2 and 3 for 37°C and columns 5 and 6 for 30°C) show stronger bands compared to the uninduced control. Thus, the promoter is slightly leaky, but the addition of proper amounts of IPTG increases protein production. When comparing temperatures, incubation at 30°C clearly yields a higher amount of RFP, but yields similar amounts of expression between both concentrations.</p> | ||
| + | |||
| + | <h2>Characterising <i>lac</i> promoter repression by glucose in different pH</h2> | ||
| + | |||
| + | <p>[https://2019.igem.org/Team:Hong_Kong_HKUST Hong_Kong_HKUST 2019] measured the activity of promoter using a GFP reporter in pH 5-9 when repressed by 0.1M glucose. This is done to assess the degree of control over activity that external factors such as pH have.</p> | ||
| + | |||
| + | <p>BBa_K741002 (p<i>Lac</i>-RBS-GFP-T construct) containing BBa_E0040 GFP is used in the experiment. Cells transformed with this part are mixed into LB of pH range 5-9. 0.1M Glucose is then added to them. A control without the addition of glucose is also included in this experiment. There is a leaked expression of pLac observed in this construct. The cell samples with and without glucose inducer are treated in the LB for the same duration. The OD and fluorescence of these samples are then measured using a plate reader.</p> | ||
| + | |||
| + | <p>pLac on its own shows leak expression while that with added glucose shows repressed activity. On plotting a graph of fluorescence/OD against pH, it was observed that the overall trend of GFP expression decreases with an increase in pH. This can be visualized in the graph of repression (difference between leak expression and glucose-induced expression) against pH.</p> | ||
| + | |||
| + | {|- | ||
| + | |[[File:T--Hong_Kong_HKUST--oldpart1.png|280px]] | ||
| + | |[[File:T--Hong_Kong_HKUST--oldpart2.png|280px]] | ||
| + | |[[File:T--Hong_Kong_HKUST--oldpart3.png|280px]] | ||
| + | |} | ||
| + | |||
| + | <p>The numerical results are tabulated as follows:</p> | ||
| + | <p>[[File:T--Hong_Kong_HKUST--oldpart4.png|300px]] | ||
| + | |||
| + | ==Characterization of expression in different sugars mixtures== | ||
| + | |||
| + | <html> | ||
| + | <p> | ||
| + | See more results on <a style="color:#3BB9FF;" href=« https://2019.igem.org/Team:Nantes/Results">our wiki page</a>. | ||
| + | </p> | ||
| + | <P> We used for BBa_K2991013 the characterization </p> | ||
| + | <br> | ||
| + | <div> | ||
| + | <br> | ||
| + | <h1 style="text-align:center;">I - Testing of the functionality of our designed parts</h1> | ||
| + | <br> | ||
| + | <p>After transforming K12 MG1655 <span class="italic">E.coli</span> with our pLAC-GFP construct, we measured by spectrofluorometry the fluorescence of the transformed bacteria cultivated in M9 medium in the presence of saturating concentration | ||
| + | (0.2%) of the associated sugar. The fluorescence studied in the cases below has been normalized with the Optical Density (OD).</p> | ||
| + | <br> | ||
| + | <div> | ||
| + | <p>Our spectrofluorometric measurements were carried out on the transformed bacteria cultivated in a M9 medium in 2 different conditions : with 0.2% of the specific sugar, and without sugar.</p> | ||
| + | <div> | ||
| + | <br> | ||
| + | <h4 style="text-decoration: underline;"> ● pLAC-GFP: <span class="italic">E.coli</span>: </h4> | ||
| + | <img class="imagegraph" src="https://2019.igem.org/wiki/images/2/2b/T--Nantes--graph1.png" alt=""> | ||
| + | <p><span class="bold">Figure 1:</span> GFP Fluorescence normalized with OD for <span class="italic">E.coli</span> K12 MG1655 transformed with pLAC in the presence (green) or absence (grey) of Lactose at 0,2%. Experiments were conducted during 17 hours.</p> | ||
| + | <p> | ||
| + | The activity of pLAC is relative to the expression of GFP fluorescence. In the condition without sugar we observe little increase in GFP fluorescence and therefore little activity of the pLAC promoter. This control shows that there is very little promoter | ||
| + | activity in the absence of sugar. This validates our control. In the condition where 0.2% lactose was added, we observe significant GFP levels compared to the condition without sugar starting at hour 1. The level of GFP increases linearly | ||
| + | over time. The reporter gene is expressed following the activation of the pLAC promoter in the presence of lactose. We observe an increase in GFP during the first 6 hours of the bacterial culture, but from the sixth hour this increase | ||
| + | slows down. This verifies the proper functioning of our designed pLAC promoter. | ||
| + | |||
| + | </p> | ||
| + | </div> | ||
| + | <br> | ||
| + | |||
| + | <div> | ||
| + | <h2 style="text-align:center;">Summary</h2> | ||
| + | <p> | ||
| + | pLAC is significantly activated in the presence of lactose compared to conditions without sugar, verifying the proper functioning of this part. | ||
| + | </p> | ||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
| + | <br> | ||
| + | <!--Partie 2--> | ||
| + | <br> | ||
| + | <div> | ||
| + | <h1 style="text-align:center;">II - The link between sugar concentration and promoter activity</h1> | ||
| + | <br> | ||
| + | <p>For these tests we measured the fluorescence in K12 MG1655 <span class="italic">E.coli</span> transformed with each of our pLAC-GFP construct. The bacteria were cultivated in M9 medium with various different concentrations of the associated sugar. The fluorescence studied in the graphs below has been normalised with the Optical Density (OD). | ||
| + | </p> | ||
| + | <br> | ||
| + | <h4 style="text-decoration: underline;"> ● pLAC-GFP <span class="italic">E.coli</span> :</h4> | ||
| + | <img class="imagegraph" style="margin-left:10%;width:80%; height:80%;" src="https://2019.igem.org/wiki/images/e/e8/T--Nantes--graph9.png" alt=""> | ||
| + | <p><span class="bold">Figure 9:</span> GFP Fluorescence normalised with OD for <span class="italic">E.coli</span> K12 MG1655 transformed with pLAC in selected concentrations of Lactose</p> | ||
| + | <p> | ||
| + | The activity of pLAC is relative to the expression of GFP fluorescence. We observe a slight increase of GFP fluorescence in the condition without sugar, but the fluorescence level remains relatively insignificant when compared to the fluorescence in conditions | ||
| + | where lactose is present. However we do not observe a significant difference of GFP expression between the different conditions containing lactose. The concentration of lactose does not seem to have a significant effect on the activity | ||
| + | of our designed pLAC in the simple insert construct. However for this conclusion to be decisive we would need to carry out more experiments with different sugar concentrations | ||
| + | |||
| + | |||
| + | </p> | ||
| + | </div> | ||
| + | <br> | ||
| + | <div> | ||
| + | <h2 style="text-align:center;">Summary</h2> | ||
| + | <p> | ||
| + | In our simple insert construct, pLAC, a high level of activation was rapidly reached with a low concentration of it cognate sugars. | ||
| + | </p> | ||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
| + | <!--Partie 3--> | ||
| + | <div> | ||
| + | <br><br> | ||
| + | |||
| + | <h1 style="text-align:center;">III - The specificity of each promoter to their associated sugar</h1> | ||
| + | <br> | ||
| + | <p>To complete our experiments we tested the promoter activity of the simple insert construct pLAC-GFP, in K12 MG1655 <span class="italic">E.coli</span>, in the presence of saturation concentration of different sugars.The primary purpose of these measurements is to confirm already published cross-activation results. | ||
| + | </p> | ||
| + | <br> | ||
| + | <div> | ||
| + | <h4 style="text-decoration: underline;"> ● pLAC-GFP <span class="italic">E.coli</span> :</h4> | ||
| + | <img class="imagegraph" style="margin-left:10%;width:80%; height:80%;" src="https://2019.igem.org/wiki/images/f/fd/T--Nantes--graph17.png" alt=""> | ||
| + | <p><span class="bold">Figure 17:</span> GFP fluorescence normalized with the OD in <span class="italic">E.coli</span> K12 MG1655 bacteria transformed with pLAC-GFP construct in the presence of saturating amounts of different sugars</p> | ||
| + | <p>The activity of pLAC is relative to the expression of GFP fluorescence. | ||
| + | <br> In absence of any sugar, we observe no increase of GFP fluorescence during the 15 hours of culture . This validates our negative control. | ||
| + | <br>On the other hand, all tested sugars were able to induce an increase of GFP fluorescence hence activate the pLAC promoter. This corroborates already published results. At this stage we cannot conclude on the differences between the | ||
| + | sugars regarding the fluorescence levels reached after 5, 10 or 15 hours since we did not quantify the amount of proteins and only normalized the data with bacterial growth OD values. | ||
| + | |||
| + | </p> | ||
| + | </div> | ||
| + | |||
| + | <div> | ||
| + | <h2 style="text-align:center;">Summary</h2> | ||
| + | <p> | ||
| + | <br> pLAC seems to be activated by all 4 sugars. These confirm cross-activation data published previously by Guy Aidelberg et al <span class="exposant">(1)</span>. | ||
| + | </p> | ||
| + | </div> | ||
| + | </div> | ||
| + | <br><br> | ||
| + | <!-- Partie 5 --> | ||
| + | <div> | ||
| + | <h1 style="text-align:center;">IV - Binary combinations of sugars with simple and double inserts</h1> | ||
| + | <p>Here we tested the activities of single insert with binary combinations of sugars. The tested single insert pLAC-GFP. The primary purpose of this experiment is to analyse the activity of pLAC promoter in the presence of 2 sugars at a time: it’s own specific sugar and another. We will see if the sugar-hierarchy is still present in our construction in this condition, and how a sugar, higher or lower in the hierarchy impacts the expression of the promoter.</p> | ||
| + | |||
| + | <br> | ||
| + | <div> | ||
| + | <h4 style="text-decoration: underline;"> ● pLAC-GFP <span class="italic">E.coli</span> :</h4> | ||
| + | <p> | ||
| + | As a reminder, here <span class="italic">E.coli</span> K12 MG1655 was transformed with pLAC-GFP. We monitored pLAC activity by measuring GFP fluorescence in absence or presence of combinations of sugars. Results are provided below (Fig. 22) . | ||
| + | </p> | ||
| + | <img class="imagegraph" style="margin-left:10%;width:80%; height:80%;" src="https://2019.igem.org/wiki/images/d/d3/T--Nantes--graph22.png" alt=""> | ||
| + | <p><span class="bold">Figure 22:</span> Figure 22. GFP Fluorescence normalized with OD for <span class="italic">E.coli</span> K12 MG1655 transformed with pLAC-GFP in absence of any sugar (■), in presence of 0.2% lactose combined with M9 rich medium (●), 0.2% arabinose (○), | ||
| + | 0.2% sorbitol (△) and 0.2% ribose (+). Experiments were conducted during 17 hours</p> | ||
| + | <p>Our results show that pLAC promoter is activated by all tested binary combinations of sugars which all contained 0.2% lactose. The increase in activity over time is mainly imputable to lactose. Addition of other sugars known to be lower in the | ||
| + | hierarchy with respect to lactose did not induced an additional effect on pLAC activity. This confirms that pLAC is on the top of the hierarchy | ||
| + | </p> | ||
| + | </div> | ||
| + | <br> | ||
| + | |||
| + | <div> | ||
| + | <h2style="text-align:center;">Summary</h2> | ||
| + | <p> | ||
| + | <br>We can conclude that in the simple construct, a sugar higher up in the hierarchy will inhibit promoters associated to sugars lower in the hierarchy. . </p> | ||
| + | </div> | ||
| + | </div> | ||
| + | |||
| + | |||
| + | |||
| + | <!--Partie 6--> | ||
| + | <div> | ||
| + | <br><br> | ||
| + | |||
| + | <h1 style="text-align:center;">V - Duration of the promoter expression</h1> | ||
| + | <br> | ||
| + | <p> | ||
| + | As our tool is mainly based on controlling gene expression in time, it is important for us to study the activity of this promoter pLACs in time. This will allow us to better understand the behavior of our promoter, enabling us to take into account any time differences we might discover in the design of our tool. | ||
| + | </p> | ||
| + | <br> | ||
| + | <div> | ||
| + | <h4 style="text-decoration: underline;"> ● pLAC-GFP <span class="italic">E.coli</span> :</h4> | ||
| + | <img class="imagegraph" style="margin-left:10%;width:80%; height:80%;" <img class="imagegraph" style="margin-left:10%;width:80%; height:80%;" src="https://2019.igem.org/wiki/images/9/90/T--Nantes--graph27.jpg"alt=""> | ||
| + | <p><span class="bold">Figure 27:</span> Rate of GFP Fluorescence/hours for E.coli K12 MG1655 transformed with pLAC-GFP simple insert in the absence or in the presence of 0,2% of Lactose</p> | ||
| + | <p> | ||
| + | The activity of pLAC is relative to the expression of GFP fluorescence.<br> | ||
| + | We do not observe the production of GFP fluorescence in the condition without sugar. It confirms our control. With 0,2% of lactose, we can observe a linear increase of GFP production per hour that begins around hour 2,5. At the 5th hour We observe a peak of production, and then the production of GFP sharply decreases until the 7th hour. <br> | ||
| + | We can conclude that the activity of the promoter pLAC, observed here as the rate of GFP production, starts 2,5 hours after the presence of sugar in the medium and it’s activity lasts around 4,5 hours. | ||
| + | |||
| + | |||
| + | </p> | ||
| + | </div> | ||
| + | |||
| + | |||
| + | <div> | ||
| + | <h2 style="text-align:center;">Summary</h2> | ||
| + | <p> | ||
| + | <br> We can conclude that the activation and the activity of pLAC vary in time, from one to the other. In saturated sugar conditions, pLAC is activated around 2,5 hours after the addition of sugar and it’s activity lasts for around 4,5 hours. <br> | ||
| + | This information would need to be taken into account to increase the precision of sequential gene expression in time of our tool. | ||
| + | </p> | ||
| + | </div> | ||
| + | </div> | ||
| + | |||
| + | <div class="expopara"> | ||
| + | <p><span class="infoexposant"> | ||
| + | (1)<span class="margeleft1">“Hierarchy of non-glucose sugars in Escherichia Coli.” Aidelberg Guy et al, BMC Systems Biology., 2014 , PMID: 25539838</span> | ||
| + | |||
| + | </span></p> | ||
| + | </div> | ||
| + | </div> | ||
| + | </html> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | ==Functional Parameters: Austin_UTexas== | ||
| + | <html> | ||
| + | <body> | ||
<partinfo>BBa_R0010 parameters</partinfo> | <partinfo>BBa_R0010 parameters</partinfo> | ||
| − | <!-- --> | + | <h3><center>Burden Imposed by this Part:</center></h3> |
| + | <figure> | ||
| + | <div class = "center"> | ||
| + | <center><img src = "https://static.igem.org/mediawiki/parts/f/fa/T--Austin_Utexas--no_burden_icon.png" style = "width:160px;height:120px"></center> | ||
| + | </div> | ||
| + | <figcaption><center><b>Burden Value: 1.9 ± 39.8% </b></center></figcaption> | ||
| + | </figure> | ||
| + | <p> Burden is the percent reduction in the growth rate of <i>E. coli</i> cells transformed with a plasmid containing this BioBrick (± values are 95% confidence limits). This BioBrick did not exhibit a burden that was significantly greater than zero (i.e., it appears to have little to no impact on growth). Therefore, users can depend on this part to remain stable for many bacterial cell divisions and in large culture volumes. Refer to any one of the | ||
| + | <a href="https://parts.igem.org/Part:BBa_K3174002">BBa_K3174002</a> - <a href="https://parts.igem.org/Part:BBa_K3174007">BBa_K3174007</a> pages for more information on the methods, an explanation of the sources of burden, and other conclusions from a large-scale measurement project conducted by the <a href="http://2019.igem.org/Team:Austin_UTexas">2019 Austin_UTexas team</a>.</p> | ||
| + | <p>This functional parameter was added by the <a href="https://2020.igem.org/Team:Austin_UTexas/Contribution">2020 Austin_UTexas team.</a></p> | ||
| + | |||
| + | |||
| + | </body> | ||
| + | </html> | ||
| + | |||
| + | ==Characterization of lac operon repression under different concentration glucose with different concentration of IPTG: HongKong-UCCKE== | ||
| + | |||
| + | <html> | ||
| + | <style> | ||
| + | td { | ||
| + | border: 1px solid #000; | ||
| + | } | ||
| + | </style> | ||
| + | <body> | ||
| + | <!--<partinfo>BBa_R0010</partinfo>--> | ||
| + | |||
| + | <p><a href="https://2024.igem.wiki/hongkong-uccke/"> HongKong-UCCKE 2024</a> investigated the effects of glucose and IPTG concentrations on <i>lac operon</i> activity in <i>E. coli</i>, to further optimize the gene expression levels of Lac promoter<i>. </i>A RFP reporter was used to measure the activity of the promoter under induction of glucose and suppression of IPTG. </p> | ||
| + | <br></br> | ||
| + | <p>RFP coding device (J04450) is expressed under Lac promoter. The transformed cells are cultured in different culture mediums for 2 sets of experiments. </p> | ||
| + | <br></br> | ||
| + | <p>Two experiments are performed: </p> | ||
| + | <ol> | ||
| + | <li>Fixed concentration of glucose (20g/L), different concentration of IPTG</li> | ||
| + | </ol> | ||
| + | <p>Single colonies are cultured in 1mL of LB(chL), whose final volume contains 20g/L of glucose. Then IPTG is added in a range of 0mM, 1mM, 1.5mM, 2mM, 5mM to each respective culture medium. </p> | ||
| + | <br></br> | ||
| + | <ol> | ||
| + | <li>Different concentration of glucose, fixed concentration of IPTG (1mM)</li> | ||
| + | </ol> | ||
| + | <p>Single colonies are cultured in 1mL of LB(chL), whose final volume contains 1mM of IPTG. Then glucose is added in a range of 0g/L, 4g/L, 8g/L, 12g/L, 16g/L, 20g/L to each respective culture medium. </p> | ||
| + | <br></br> | ||
| + | <p>Samples are incubated for 4 hours and 24 hours, with OD<sub>600</sub> and fluorescence measured using a plate reader respectively. </p> | ||
| + | <br></br> | ||
| + | <p>Results </p> | ||
| + | <br></br> | ||
| + | <p>Investigation of promoter expression level in 4 hour and 24 hour incubation </p> | ||
| + | <table> | ||
| + | <tr> | ||
| + | <td> | ||
| + | <p>Under 4 hour incubation </p> | ||
| + | </td> | ||
| + | <td> | ||
| + | <p>Under 24 hour incubation </p> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td> | ||
| + | <img src="https://static.igem.wiki/teams/5201/parts-registry/part-registry-1.png" style = "width:30vw" style = "width:30vw" ></img> | ||
| + | <br></br> | ||
| + | <p>(Part_registry_1)</p> | ||
| + | </td> | ||
| + | <td> | ||
| + | <img src="https://static.igem.wiki/teams/5201/parts-registry/part-registry-2.png" style = "width:30vw" style = "width:30vw" ></img> | ||
| + | <br></br> | ||
| + | <p>(Part_registry_2)</p> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td colspan="2"> | ||
| + | <p>Figs. 1, 2 Fluorescence per bacterial concentration (OD<sub>600</sub>) against different glucose concentration with fixed IPTG concentration after 4 hours and 24 hours incubation</p> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <p>The inhibitory effect of glucose on <i>lac operon</i> expression was observed after 24 hour incubation. In the initial 4 hours, fluorescence/OD<sub>600</sub> across all glucose concentrations showed minimum difference. Under 24 hour incubation, suppressive effects of glucose are noticeable. </p> | ||
| + | <br></br> | ||
| + | <br></br> | ||
| + | <table> | ||
| + | <tr> | ||
| + | <td> | ||
| + | <img src="https://static.igem.wiki/teams/5201/parts-registry/part-registry-3.png" style = "width:30vw" ></img> | ||
| + | <p>(Part_registry_3)</p> | ||
| + | <p>Fig. 3 OD<sub>600</sub> against different glucose concentration with fixed IPTG concentration after 4-hour incubation</p> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <p>From observing the graph OD<sub>600</sub> against different concentrations of glucose under fixed IPTG, the OD<sub>600</sub> across all glucose concentrations is similar. Therefore, glucose concentration does not affect the growth of <i>E. coli</i>, but only the gene expression level. </p> | ||
| + | <br></br> | ||
| + | <br></br> | ||
| + | <table> | ||
| + | <tr> | ||
| + | <td> | ||
| + | <p>Different glucose, fixed IPTG </p> | ||
| + | </td> | ||
| + | <td> | ||
| + | <p>Fixed glucose, different IPTG </p> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td> | ||
| + | <img src="https://static.igem.wiki/teams/5201/parts-registry/part-registry-4.png" style = "width:30vw" ></img> | ||
| + | <p>(Part_registry_4)</p> | ||
| + | </td> | ||
| + | <td> | ||
| + | <img src="https://static.igem.wiki/teams/5201/parts-registry/part-registry-5.png" style = "width:30vw" ></img> | ||
| + | <p>(Part_registry_5)</p> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td colspan="2"> | ||
| + | <p>Figs. 4, 5 Fluorescence per OD<sub>600</sub> against different IPTG in 20g/L glucose after overnight incubation</p> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <p>Fluorescence/OD<sub>600</sub> in 0g/L glucose is the highest. While glucose added culture media has a low fluorescence/OD<sub>600</sub>. Any amount of glucose concentration significantly affects the gene expression level, no matter how much IPTG is applied for induction.</p> | ||
| + | <br></br> | ||
| + | <br></br> | ||
| + | <br></br> | ||
| + | <br></br> | ||
| + | |||
| + | |||
| + | </body> | ||
| + | </html> | ||
Latest revision as of 10:19, 2 October 2024
promoter (lacI regulated)
This part is an inverting regulator sensitive to LacI and CAP.
It contains two protein binding sites. The first binds the CAP protein, which is generally present in E.coli and is asocciated with cell health and availability of glucose. The second binds LacI protein.
- In the absence of LacI protein and CAP protein, this part promotes transcription.
- In the presence of LacI protein and CAP protein, this part inhibits transcription.
- LacI can be inhibited by [http://openwetware.org/wiki/IPTG IPTG].
- LacI is coded by BBa_C0010
Intrinsic noise value: 0.0707 (compare with R0011: 0.0040; R0051: 0.0869). See [http://2015.igem.org/Team:William_and_Mary William_and_Mary iGEM 2015]
>Internal Priming Screening Characterization of BBa_R0010: Has 3 possible internal priming site between this BioBrick part and the VR primer.
The 2018 Hawaii iGEM team evaluated the 40 most frequently used BioBricks and ran them through an internal priming screening process that we developed using the BLAST program tool. Out of the 40 BioBricks we evaluated, 10 of them showed possible internal priming of either the VF2 or VR primers and sometime even both. The data set has a range of sequence lengths from as small as 12 bases to as large as 1,210 bases. We experienced the issue of possible internal priming during the sequence verification process of our own BBa_K2574001 BioBrick and in the cloning process to express the part as a fusion protein. BBa_K2574001 is a composite part containing a VLP forming Gag protein sequence attached to a frequently used RFP part (BBa_E1010). We conducted a PCR amplification of the Gag-RFP insert using the VF2 and VR primers on the ligation product (pSB1C3 ligated to the Gag + RFP). This amplicon would serve as template for another PCR where we would add the NcoI and BamHI restriction enzyme sites through new primers for ligation into pET14b and subsequent induced expression. Despite gel confirming a rather large, approximately 2.1 kb insert band, our sequencing results with the VR primer and BamHI RFP reverse primer gave mixed results. Both should have displayed the end of the RFP, but the VR primer revealed the end of the Gag. Analysis of the VR primer on the Gag-RFP sequence revealed several sites where the VR primer could have annealed with ~9 - 12 bp of complementarity. Internal priming of forward and reverse primers can be detrimental to an iGEM project because you can never be sure if the desired construct was correctly inserted into the BioBrick plasmid without a successful sequence verification.
For the BioBrick part BBa_R0010, the first location of the internal priming site is on the 121-113 base number of the BioBrick and on the 12-20 base number of the VR primer. The second location of the internal priming site is on the 11-17 base number of the BioBrick and on the 4-10 base number of the VR primer. The third location of the internal priming site is on the 84-90 base number of the BioBrick and on the 14-20 base number of the VR primer.
Usage and Biology
This is a direct copy of bases 0365739 through 0365540 of the E. coli K-12 MG1655 genome, Genbank NC_000913 in reverse complement form. It is the natural promoter for the LacZYA operon. It includes the tail end of the LacI gene coding region, but no promoter region for that partial gene.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Usage in Chromobacterium Violaceum
[http://2016.igem.org/Team:Tec-Monterrey Team Tec-Monterrey 2016] tested the Promoter BBa_R0010 in C. Violaceum. We were unable to confirm whether or not C. Violaceum had the lac operon, BLAST analysis of its genome suggested that it did not have this regulating system; our characterization demonstrates that the lac promoter can be used as a constitutive promoter in this chassis. There was no significant difference in the Fluorescence of the group of C. Violaceum with IPTG or without it, but in E. Coli it showed normal induction. Therefore, we can confirm that this promoter is constitutive in C. Violaceum. 

Characterization for usage in genetically recoded organisms and expanded genetic code
Team Aboa 2019used this biobrick in production optimization ofcell strain C321.ΔA.exp using expanded genetic code in expression for modified proteins. This biobrick is completely functional and usable also for production of proteins modified with unnatural amino acids.
For the measurements we used Cytation5 in overnight incubation and shaking at 37 degrees celsius. Fluorescence was measured every 30 minutes at preset bandwith using GFP and mCherry programs. Relative fluorescence is calculated as fluorescence at the start of induction with IPTG, pAzF and arabinose divided by the end of incubation the next morning.
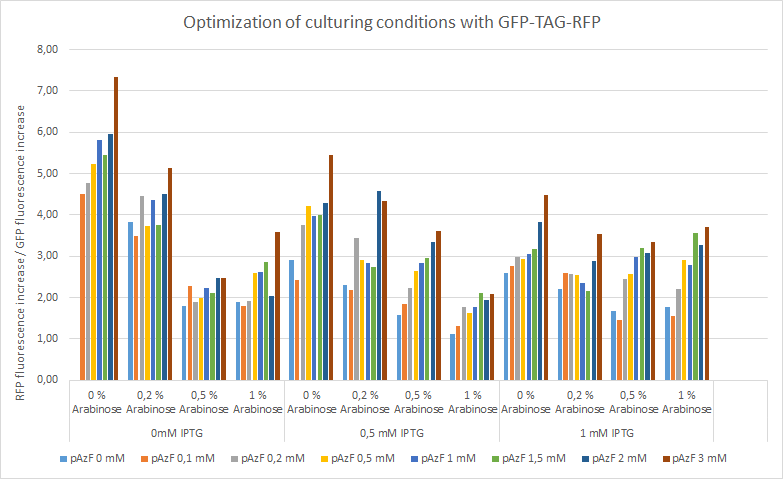
The relative change of RFP fluorescence divided by the change of GFP fluorescence after induction with variable amounts of p-azido-l-phenylalanine, IPTG and arabinose. Relative change of fluorescence is used to normalize signals that are present before induction. The figure shows that IPTG concentrations does not have an effect on the expression of RFP as the promoter is leaky and the arabinose and pAzF are much more critical for producing proteins with unnatural amino acid modifications. High relative expression when little or no pAzF or arabinose is present is most likely a sign of misincorporation of amino acids in avoidance of ribosome stalling.
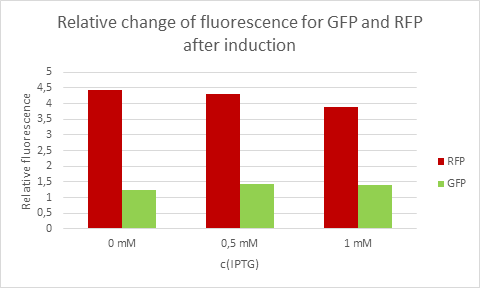
Average eelative change of fluorescence from each concentration of IPTG.
Usage of the lac promoter to express fluorescent markers in two different species
Hong_Kong_HKU tested this promoter (BBa_R0010) in two different species, Escherichia coli and Salmonella Typhimurium to test whether this is a constitutive promoter in S. Typhi.
E. coli and S. Typhimurium belong to the same order, Enterobacteriaceae. Lactose fermentation ability is used to differentiate them. S. Typhimurium does not have the ability to ferment lactose like E. coli does. In this characterisation experiment, this lac promoter is transformed to S. Typhimurium and E. coli as a control to see the difference in the expression level of the downstream red fluorescent markers.
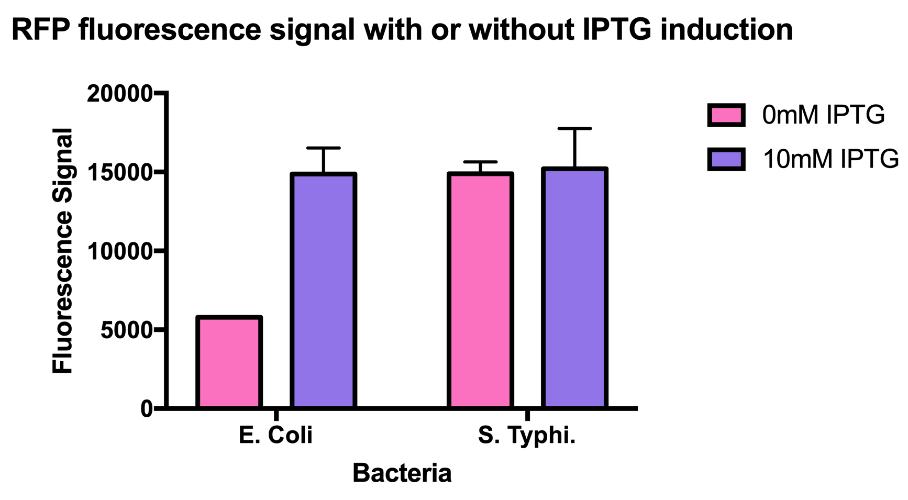
As the data suggests, this promoter is a constitutive promoter in S. Typhimurium as there is no difference in signals with or without IPTG. As expected, under IPTG induction, there is a 3-fold increase in RFP signals compared to those without IPTG.
Tec-Chihuahua 2019: Usage of lacI promoter to express RFP into E.coli SHuffle T7 Express
Team Tec-Chihuahuaevaluated the functionality of this promoter by testing it in E. coli expression strain SHuffle T7 Express®️. SHuffle was chosen as chassis for having a cytoplasmic environment that favors the production of disulfide bonds. A designed was made for the composite parts under the regulation of part BBa_R0010, a lacI regulated promoter inducible by IPTG. However,characterization of promoters in the iGEM Parts Registry for their use in SHuffle strains is nonexistent.
To evaluate protein production by part BBa_R0010, part BBa_J04450 was transformed, an RFP coding device under the regulation of this promoter, into E. coli SHuffle T7 Express®️. Transformed cells were grown on LB agar with chloramphenicol, for BBa_J04450 was located in the pSB1C3 backbone.
RFP expression was induced at different temperature conditions and IPTG concentration to determine the ideal conditions for the proper functionality of the promoter. Protein expression was analysed through an SDS-PAGE.
The functionality of promoter BBa_R0010 in E. coli SHuffle T7 Express®️ was demonstrated as well as ideal conditions for protein production by characterizing part BBa_J04450, an RFP construct. E. coli SHuffle T7 Express®️ produces higher amounts of protein at 30°C, and concentrations of 0.2 and 0.4 mM IPTG are enough to induce expression when using this lacI regulated promoter.
| Well | 37°C | Well | 30°C |
|---|---|---|---|
| Negative control | Untransformed control | Negative control | Untransformed control |
| Uninduced control (without IPTG) | Uninduced control (without IPTG) | ||
| |
Transformed cells, 0.2 mM IPTG | |
Transformed cells, 0.2 mM IPTG |
| |
Transformed cells, 0.4 mM IPTG | |
Transformed cells, 0.4 mM IPTG |
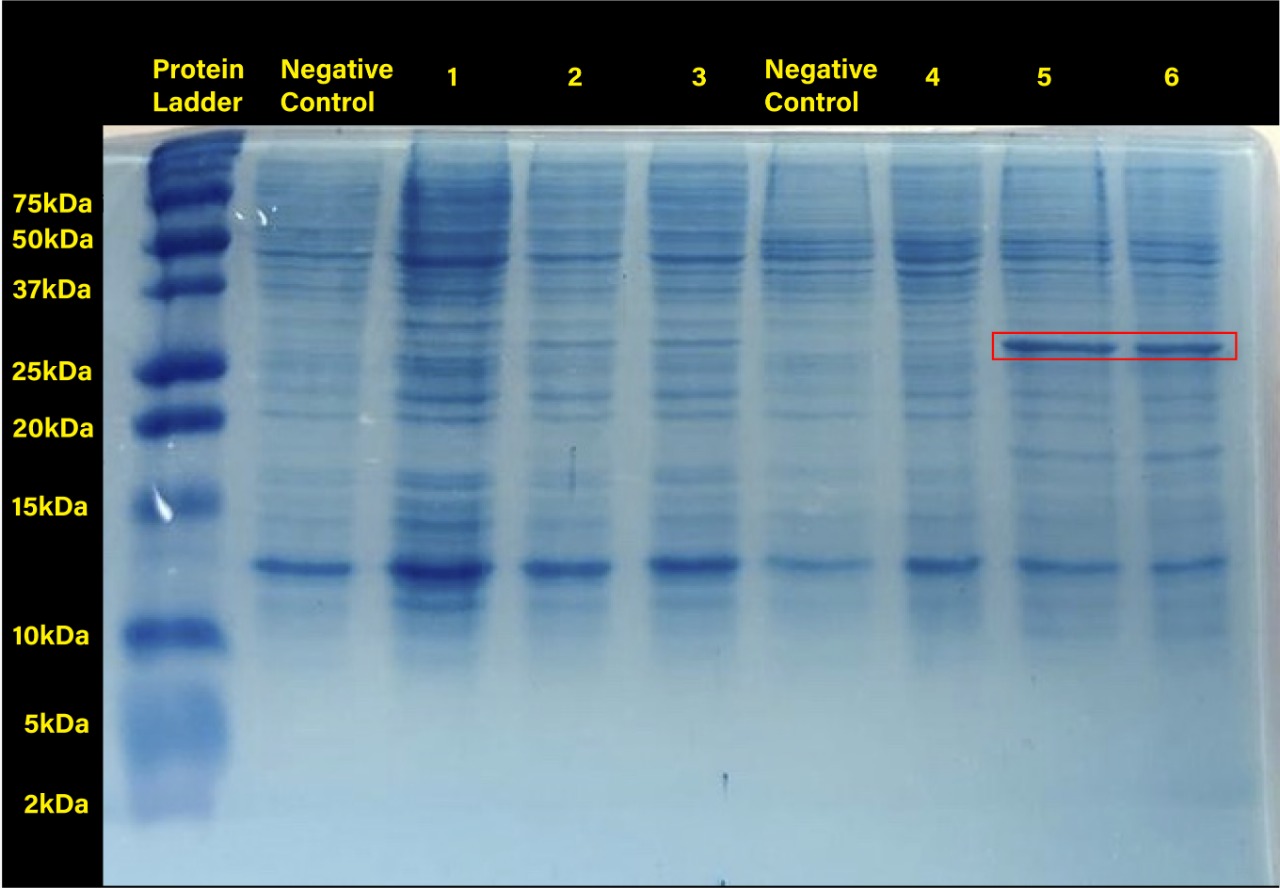
Two prominent bands can be appreciated in the last two columns, corresponding to induction conditions of 0.2 and 0.4 mM IPTG at 30°C, close to the 25 kDa band of the Precision Plus ProteinTM Dual Xtra Prestained Protein Standards. It was concluded that they correspond to RFP, a 26 kDa protein.
When taking a closer look, the band corresponding to RFP can be seen in each column with exception of the untransformed cells controls (negative controls). The uninduced controls (column 1 for 37°C and column 4 for 30°C) without IPTG were expected not to show bands if the promoter lacked leakiness; nevertheless, a faint band can be seen for both controls. Bands corresponding to 0.2 and 0.4 mM IPTG for both temperatures (columns 2 and 3 for 37°C and columns 5 and 6 for 30°C) show stronger bands compared to the uninduced control. Thus, the promoter is slightly leaky, but the addition of proper amounts of IPTG increases protein production. When comparing temperatures, incubation at 30°C clearly yields a higher amount of RFP, but yields similar amounts of expression between both concentrations.
Characterising lac promoter repression by glucose in different pH
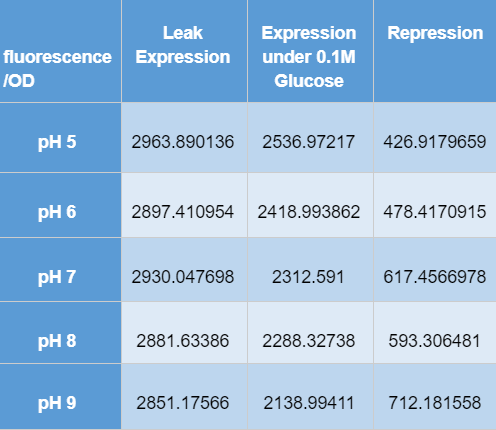
Hong_Kong_HKUST 2019 measured the activity of promoter using a GFP reporter in pH 5-9 when repressed by 0.1M glucose. This is done to assess the degree of control over activity that external factors such as pH have.
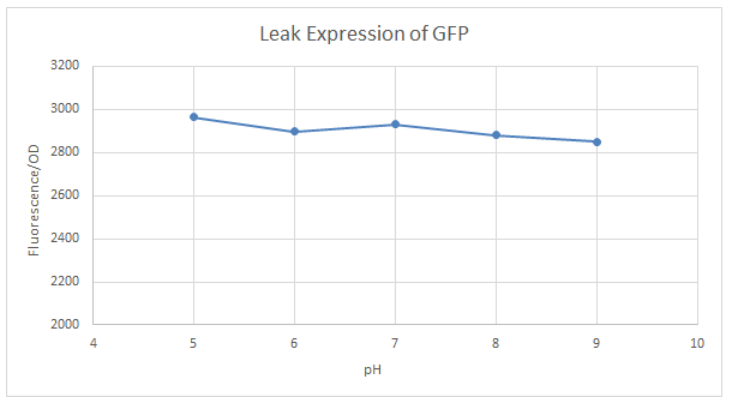
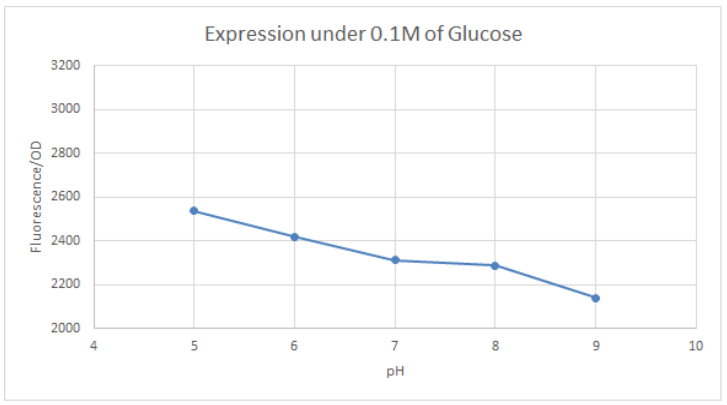
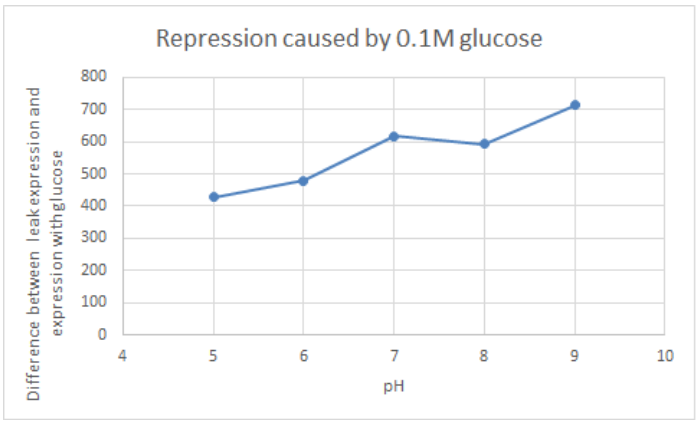
BBa_K741002 (pLac-RBS-GFP-T construct) containing BBa_E0040 GFP is used in the experiment. Cells transformed with this part are mixed into LB of pH range 5-9. 0.1M Glucose is then added to them. A control without the addition of glucose is also included in this experiment. There is a leaked expression of pLac observed in this construct. The cell samples with and without glucose inducer are treated in the LB for the same duration. The OD and fluorescence of these samples are then measured using a plate reader.
pLac on its own shows leak expression while that with added glucose shows repressed activity. On plotting a graph of fluorescence/OD against pH, it was observed that the overall trend of GFP expression decreases with an increase in pH. This can be visualized in the graph of repression (difference between leak expression and glucose-induced expression) against pH.
|
|
|
The numerical results are tabulated as follows:
Characterization of expression in different sugars mixtures
See more results on our wiki page.
We used for BBa_K2991013 the characterization
I - Testing of the functionality of our designed parts
After transforming K12 MG1655 E.coli with our pLAC-GFP construct, we measured by spectrofluorometry the fluorescence of the transformed bacteria cultivated in M9 medium in the presence of saturating concentration (0.2%) of the associated sugar. The fluorescence studied in the cases below has been normalized with the Optical Density (OD).
Our spectrofluorometric measurements were carried out on the transformed bacteria cultivated in a M9 medium in 2 different conditions : with 0.2% of the specific sugar, and without sugar.
● pLAC-GFP: E.coli:
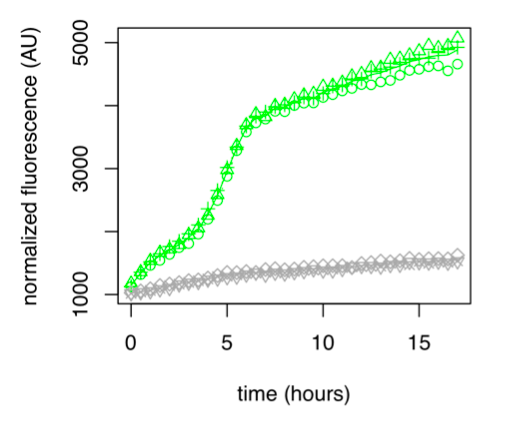
Figure 1: GFP Fluorescence normalized with OD for E.coli K12 MG1655 transformed with pLAC in the presence (green) or absence (grey) of Lactose at 0,2%. Experiments were conducted during 17 hours.
The activity of pLAC is relative to the expression of GFP fluorescence. In the condition without sugar we observe little increase in GFP fluorescence and therefore little activity of the pLAC promoter. This control shows that there is very little promoter activity in the absence of sugar. This validates our control. In the condition where 0.2% lactose was added, we observe significant GFP levels compared to the condition without sugar starting at hour 1. The level of GFP increases linearly over time. The reporter gene is expressed following the activation of the pLAC promoter in the presence of lactose. We observe an increase in GFP during the first 6 hours of the bacterial culture, but from the sixth hour this increase slows down. This verifies the proper functioning of our designed pLAC promoter.
Summary
pLAC is significantly activated in the presence of lactose compared to conditions without sugar, verifying the proper functioning of this part.
II - The link between sugar concentration and promoter activity
For these tests we measured the fluorescence in K12 MG1655 E.coli transformed with each of our pLAC-GFP construct. The bacteria were cultivated in M9 medium with various different concentrations of the associated sugar. The fluorescence studied in the graphs below has been normalised with the Optical Density (OD).
● pLAC-GFP E.coli :
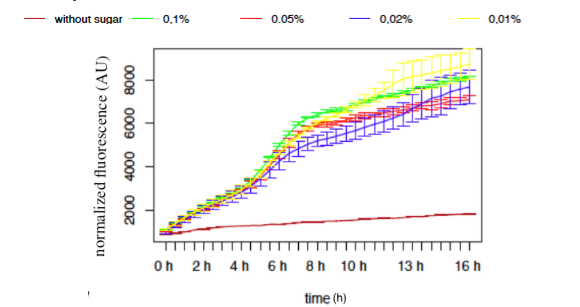
Figure 9: GFP Fluorescence normalised with OD for E.coli K12 MG1655 transformed with pLAC in selected concentrations of Lactose
The activity of pLAC is relative to the expression of GFP fluorescence. We observe a slight increase of GFP fluorescence in the condition without sugar, but the fluorescence level remains relatively insignificant when compared to the fluorescence in conditions where lactose is present. However we do not observe a significant difference of GFP expression between the different conditions containing lactose. The concentration of lactose does not seem to have a significant effect on the activity of our designed pLAC in the simple insert construct. However for this conclusion to be decisive we would need to carry out more experiments with different sugar concentrations
Summary
In our simple insert construct, pLAC, a high level of activation was rapidly reached with a low concentration of it cognate sugars.
III - The specificity of each promoter to their associated sugar
To complete our experiments we tested the promoter activity of the simple insert construct pLAC-GFP, in K12 MG1655 E.coli, in the presence of saturation concentration of different sugars.The primary purpose of these measurements is to confirm already published cross-activation results.
● pLAC-GFP E.coli :
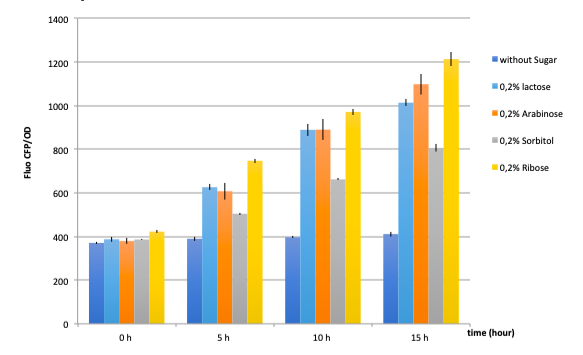
Figure 17: GFP fluorescence normalized with the OD in E.coli K12 MG1655 bacteria transformed with pLAC-GFP construct in the presence of saturating amounts of different sugars
The activity of pLAC is relative to the expression of GFP fluorescence.
In absence of any sugar, we observe no increase of GFP fluorescence during the 15 hours of culture . This validates our negative control.
On the other hand, all tested sugars were able to induce an increase of GFP fluorescence hence activate the pLAC promoter. This corroborates already published results. At this stage we cannot conclude on the differences between the
sugars regarding the fluorescence levels reached after 5, 10 or 15 hours since we did not quantify the amount of proteins and only normalized the data with bacterial growth OD values.
Summary
pLAC seems to be activated by all 4 sugars. These confirm cross-activation data published previously by Guy Aidelberg et al (1).
IV - Binary combinations of sugars with simple and double inserts
Here we tested the activities of single insert with binary combinations of sugars. The tested single insert pLAC-GFP. The primary purpose of this experiment is to analyse the activity of pLAC promoter in the presence of 2 sugars at a time: it’s own specific sugar and another. We will see if the sugar-hierarchy is still present in our construction in this condition, and how a sugar, higher or lower in the hierarchy impacts the expression of the promoter.
● pLAC-GFP E.coli :
As a reminder, here E.coli K12 MG1655 was transformed with pLAC-GFP. We monitored pLAC activity by measuring GFP fluorescence in absence or presence of combinations of sugars. Results are provided below (Fig. 22) .
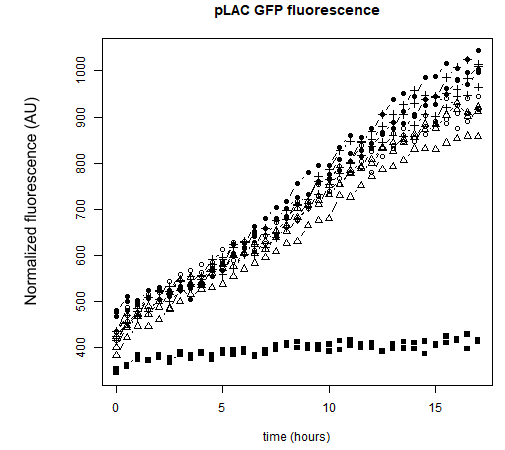
Figure 22: Figure 22. GFP Fluorescence normalized with OD for E.coli K12 MG1655 transformed with pLAC-GFP in absence of any sugar (■), in presence of 0.2% lactose combined with M9 rich medium (●), 0.2% arabinose (○), 0.2% sorbitol (△) and 0.2% ribose (+). Experiments were conducted during 17 hours
Our results show that pLAC promoter is activated by all tested binary combinations of sugars which all contained 0.2% lactose. The increase in activity over time is mainly imputable to lactose. Addition of other sugars known to be lower in the hierarchy with respect to lactose did not induced an additional effect on pLAC activity. This confirms that pLAC is on the top of the hierarchy
We can conclude that in the simple construct, a sugar higher up in the hierarchy will inhibit promoters associated to sugars lower in the hierarchy. .
V - Duration of the promoter expression
As our tool is mainly based on controlling gene expression in time, it is important for us to study the activity of this promoter pLACs in time. This will allow us to better understand the behavior of our promoter, enabling us to take into account any time differences we might discover in the design of our tool.
● pLAC-GFP E.coli :
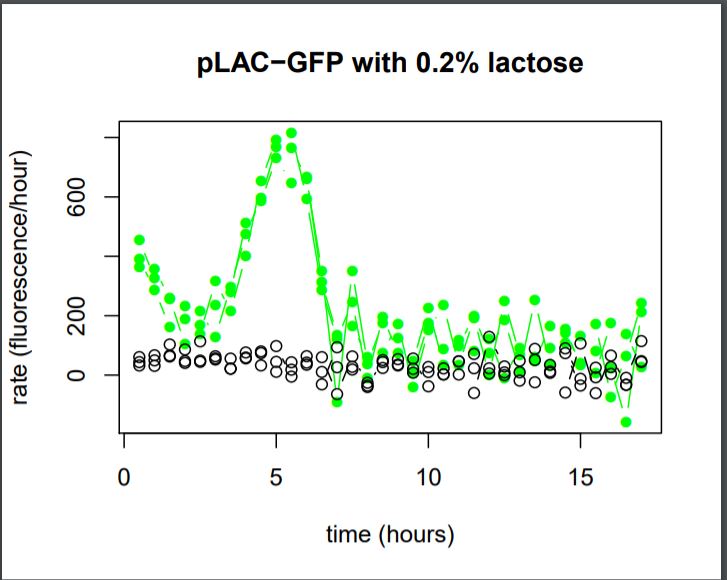
Figure 27: Rate of GFP Fluorescence/hours for E.coli K12 MG1655 transformed with pLAC-GFP simple insert in the absence or in the presence of 0,2% of Lactose
The activity of pLAC is relative to the expression of GFP fluorescence.
We do not observe the production of GFP fluorescence in the condition without sugar. It confirms our control. With 0,2% of lactose, we can observe a linear increase of GFP production per hour that begins around hour 2,5. At the 5th hour We observe a peak of production, and then the production of GFP sharply decreases until the 7th hour.
We can conclude that the activity of the promoter pLAC, observed here as the rate of GFP production, starts 2,5 hours after the presence of sugar in the medium and it’s activity lasts around 4,5 hours.
Summary
We can conclude that the activation and the activity of pLAC vary in time, from one to the other. In saturated sugar conditions, pLAC is activated around 2,5 hours after the addition of sugar and it’s activity lasts for around 4,5 hours.
This information would need to be taken into account to increase the precision of sequential gene expression in time of our tool.
(1)“Hierarchy of non-glucose sugars in Escherichia Coli.” Aidelberg Guy et al, BMC Systems Biology., 2014 , PMID: 25539838
Functional Parameters: Austin_UTexas
Burden Imposed by this Part:

Burden is the percent reduction in the growth rate of E. coli cells transformed with a plasmid containing this BioBrick (± values are 95% confidence limits). This BioBrick did not exhibit a burden that was significantly greater than zero (i.e., it appears to have little to no impact on growth). Therefore, users can depend on this part to remain stable for many bacterial cell divisions and in large culture volumes. Refer to any one of the BBa_K3174002 - BBa_K3174007 pages for more information on the methods, an explanation of the sources of burden, and other conclusions from a large-scale measurement project conducted by the 2019 Austin_UTexas team.
This functional parameter was added by the 2020 Austin_UTexas team.
Characterization of lac operon repression under different concentration glucose with different concentration of IPTG: HongKong-UCCKE
HongKong-UCCKE 2024 investigated the effects of glucose and IPTG concentrations on lac operon activity in E. coli, to further optimize the gene expression levels of Lac promoter. A RFP reporter was used to measure the activity of the promoter under induction of glucose and suppression of IPTG.
RFP coding device (J04450) is expressed under Lac promoter. The transformed cells are cultured in different culture mediums for 2 sets of experiments.
Two experiments are performed:
- Fixed concentration of glucose (20g/L), different concentration of IPTG
Single colonies are cultured in 1mL of LB(chL), whose final volume contains 20g/L of glucose. Then IPTG is added in a range of 0mM, 1mM, 1.5mM, 2mM, 5mM to each respective culture medium.
- Different concentration of glucose, fixed concentration of IPTG (1mM)
Single colonies are cultured in 1mL of LB(chL), whose final volume contains 1mM of IPTG. Then glucose is added in a range of 0g/L, 4g/L, 8g/L, 12g/L, 16g/L, 20g/L to each respective culture medium.
Samples are incubated for 4 hours and 24 hours, with OD600 and fluorescence measured using a plate reader respectively.
Results
Investigation of promoter expression level in 4 hour and 24 hour incubation
|
Under 4 hour incubation |
Under 24 hour incubation |

(Part_registry_1) |

(Part_registry_2) |
|
Figs. 1, 2 Fluorescence per bacterial concentration (OD600) against different glucose concentration with fixed IPTG concentration after 4 hours and 24 hours incubation |
|
The inhibitory effect of glucose on lac operon expression was observed after 24 hour incubation. In the initial 4 hours, fluorescence/OD600 across all glucose concentrations showed minimum difference. Under 24 hour incubation, suppressive effects of glucose are noticeable.

(Part_registry_3) Fig. 3 OD600 against different glucose concentration with fixed IPTG concentration after 4-hour incubation |
From observing the graph OD600 against different concentrations of glucose under fixed IPTG, the OD600 across all glucose concentrations is similar. Therefore, glucose concentration does not affect the growth of E. coli, but only the gene expression level.
|
Different glucose, fixed IPTG |
Fixed glucose, different IPTG |

(Part_registry_4) |

(Part_registry_5) |
|
Figs. 4, 5 Fluorescence per OD600 against different IPTG in 20g/L glucose after overnight incubation |
|
Fluorescence/OD600 in 0g/L glucose is the highest. While glucose added culture media has a low fluorescence/OD600. Any amount of glucose concentration significantly affects the gene expression level, no matter how much IPTG is applied for induction.