Difference between revisions of "Part:BBa K1033933"
(→2.SDS-PAGE verification of aspink expression) |
LucyShi 2018 (Talk | contribs) |
||
| (14 intermediate revisions by one other user not shown) | |||
| Line 6: | Line 6: | ||
'''Important: This part is not available in the registry yet, however, the same part is available from the registry with the standard RBS B0034: <partinfo>BBa_K1033927</partinfo>.''' | '''Important: This part is not available in the registry yet, however, the same part is available from the registry with the standard RBS B0034: <partinfo>BBa_K1033927</partinfo>.''' | ||
<br><br> | <br><br> | ||
| + | |||
| + | |||
| + | <!-- --> | ||
| + | <span class='h3bb'>Sequence and Features</span> | ||
| + | <partinfo>BBa_K1033933 SequenceAndFeatures</partinfo> | ||
| + | |||
===Usage and Biology=== | ===Usage and Biology=== | ||
| Line 79: | Line 85: | ||
[http://www.jbc.org/content/280/4/2401.short]Wilmann, Pascal G., et al. "Variations on the GFP Chromophore - A Polypeptide Fragmentation Within The Chromophore Revealed In The 2.1-Å Crystal Structure Of A Nonfluorescent Chromoprotein From Anemonia Sulcata." Journal of Biological Chemistry 280.4 (2005): 2401-2404. | [http://www.jbc.org/content/280/4/2401.short]Wilmann, Pascal G., et al. "Variations on the GFP Chromophore - A Polypeptide Fragmentation Within The Chromophore Revealed In The 2.1-Å Crystal Structure Of A Nonfluorescent Chromoprotein From Anemonia Sulcata." Journal of Biological Chemistry 280.4 (2005): 2401-2404. | ||
| − | == | + | ==Characterization from iGEM22_Worldshaper-Nanjing== |
Group: Worldshaper-Nanjing 2022iGEM | Group: Worldshaper-Nanjing 2022iGEM | ||
<br> | <br> | ||
| Line 88: | Line 94: | ||
| − | |||
We obtained the plasmid vector containing this part, pET-29a-aspink plasmid, from iGEM team iGEM13_Uppsala. We transformed this plasmid into E.coli strain BL21, and induced aspink expression. We extracted and purified the aspink protein by sonication, ultrafiltration, and Ni-affinity chromatography. With bioinformation analysis using Swiss Model, we predicted the structure of aspink protein and analyzed detailed characteristics of the protein. The relative molecular mass was measured by mass spectrometry. | We obtained the plasmid vector containing this part, pET-29a-aspink plasmid, from iGEM team iGEM13_Uppsala. We transformed this plasmid into E.coli strain BL21, and induced aspink expression. We extracted and purified the aspink protein by sonication, ultrafiltration, and Ni-affinity chromatography. With bioinformation analysis using Swiss Model, we predicted the structure of aspink protein and analyzed detailed characteristics of the protein. The relative molecular mass was measured by mass spectrometry. | ||
| − | ==Experiments Results== | + | ===Experiments Results=== |
| − | ===1.Induced expression of aspink in E. coli BL21 transformed with pET-29a aspink plasmid=== | + | ====1.Induced expression of aspink in E. coli BL21 transformed with pET-29a aspink plasmid==== |
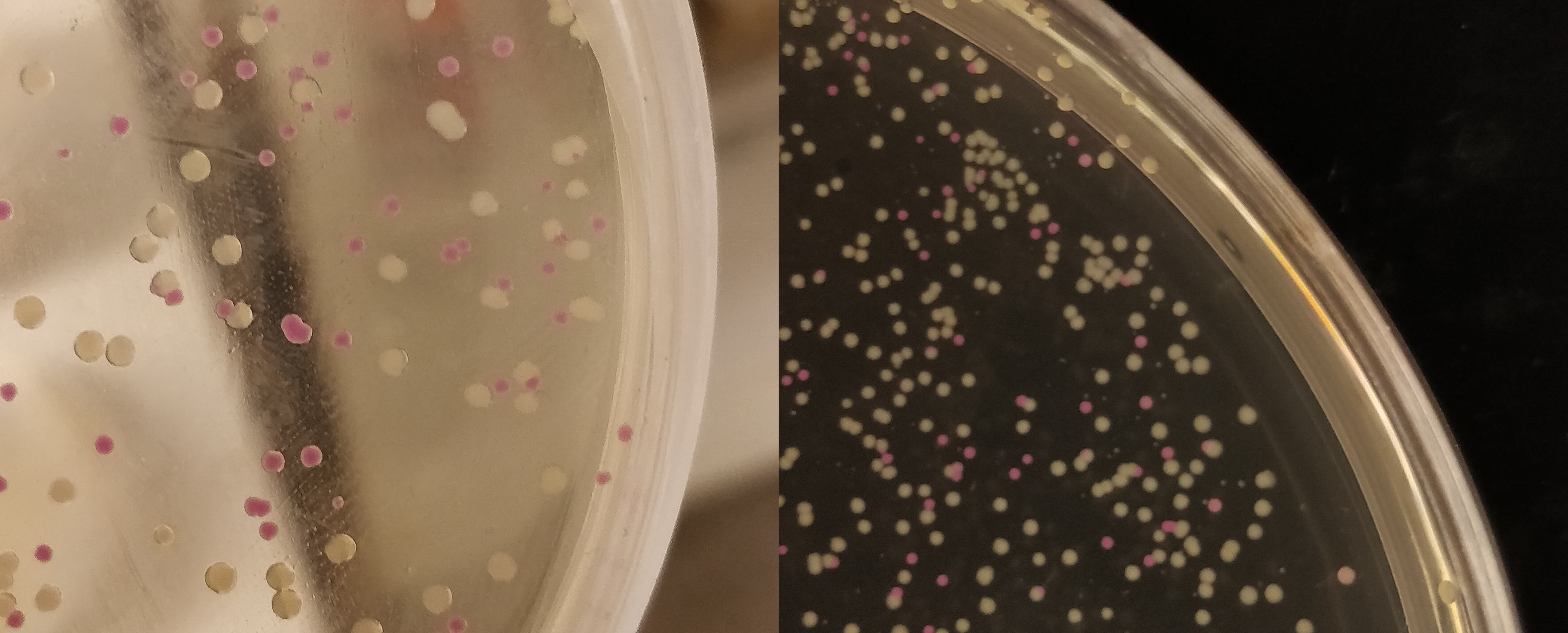
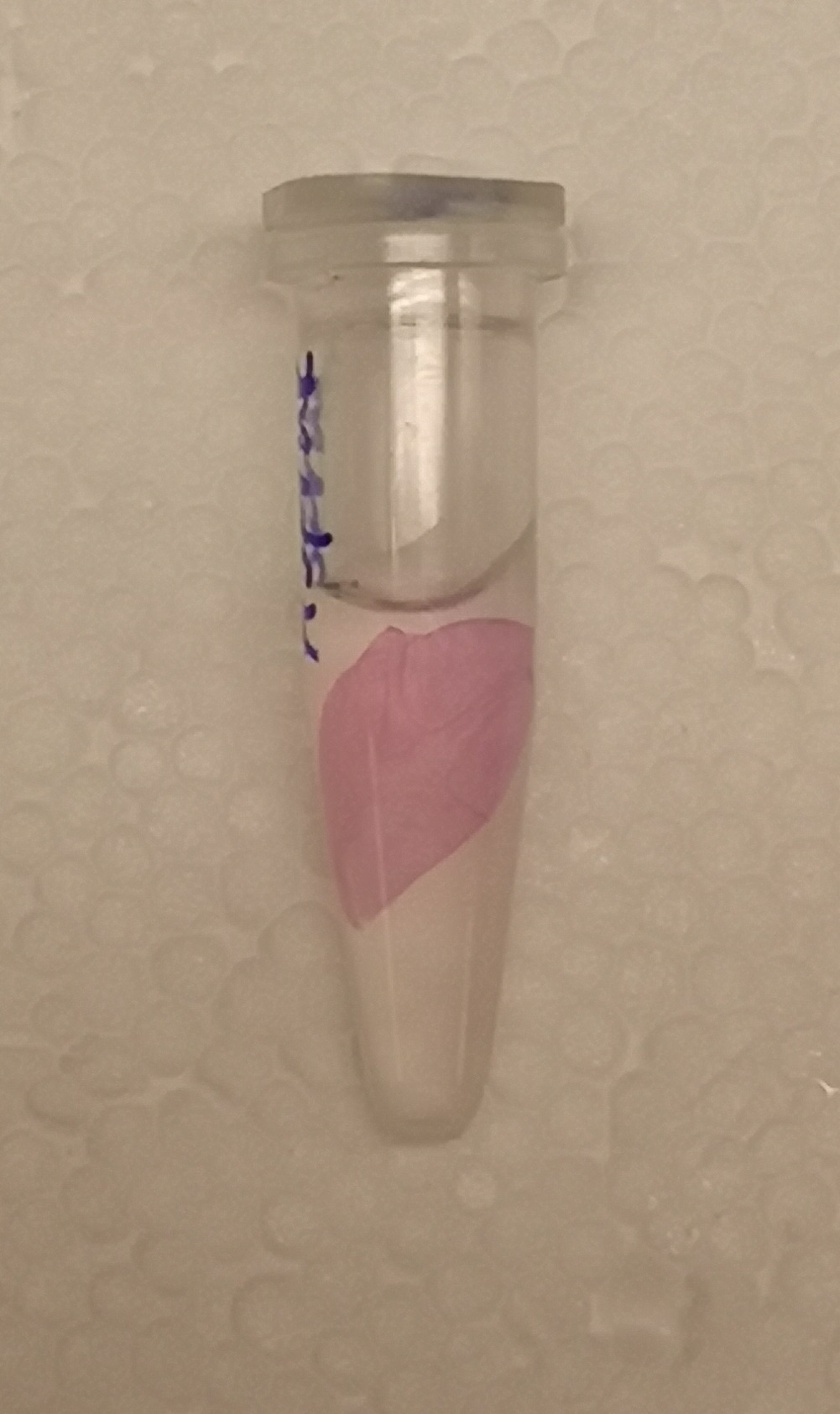
The growth performance of the BL21 bacteria samples transformed with pET-29a-aspink plasmid was measured. Two samples with OD value around 0.6 were used as the control group and the induced group, respectively. IPTG was added to induce aspink expression (Figure 1). Protein was extracted and purified from the two groups by sonication, ultrafiltration, and Ni-affinity chromatography (Figure 2). | The growth performance of the BL21 bacteria samples transformed with pET-29a-aspink plasmid was measured. Two samples with OD value around 0.6 were used as the control group and the induced group, respectively. IPTG was added to induce aspink expression (Figure 1). Protein was extracted and purified from the two groups by sonication, ultrafiltration, and Ni-affinity chromatography (Figure 2). | ||
| − | [[File:aspink-fig1.png| | + | [[File:aspink-fig1.png|400px|thumb|left|Figure 1. Comparison of induced group (left) and control group (right) for the induced expression of pET-29a-aspink plasmid in BL21]] |
| − | [[File:aspink-fig2.png|200px|thumb| | + | [[File:aspink-fig2.png|200px|thumb|center|Figure2. Purification of aspink protein with nickel column]] |
| − | ===2.SDS-PAGE verification of aspink expression=== | + | ====2.SDS-PAGE verification of aspink expression==== |
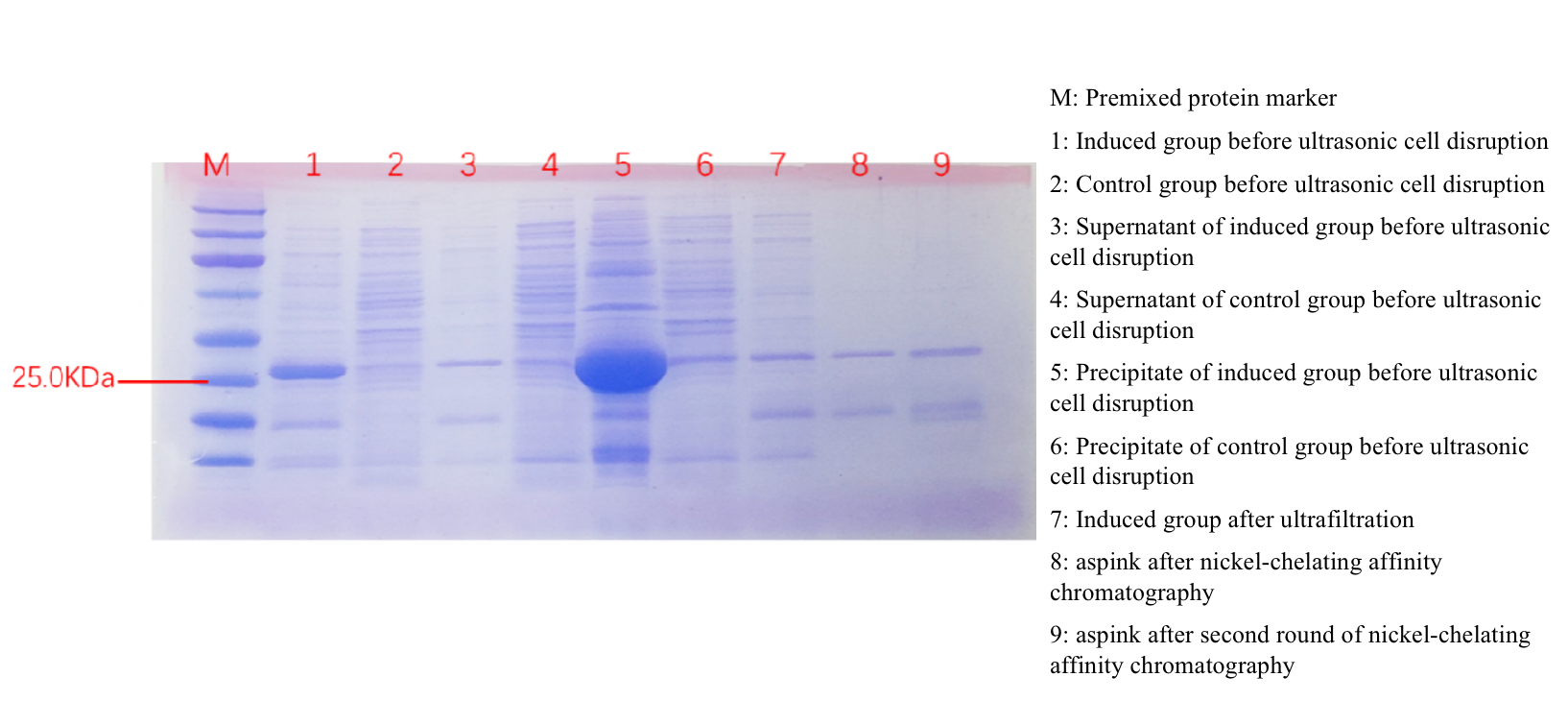
SDS-PAGE experiment verified the successful expression of aspink protein induced in BL21 (Figure 3). The size of aspink is predicted around 26 kDa. | SDS-PAGE experiment verified the successful expression of aspink protein induced in BL21 (Figure 3). The size of aspink is predicted around 26 kDa. | ||
| − | [[File:aspink-fig3.png| | + | [[File:aspink-fig3.png|800px|thumb|center|Figure 3. SDS-PAGE protein detection of aspink protein]] |
| − | + | ||
| − | + | ||
| − | + | ||
| + | ====3.Aspink Bioinformation Analysis ==== | ||
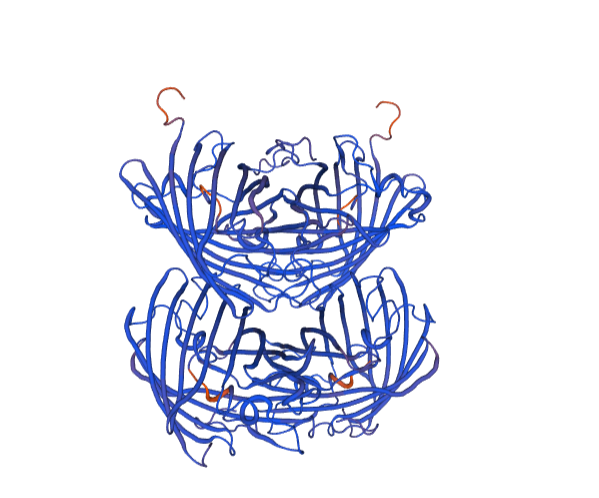
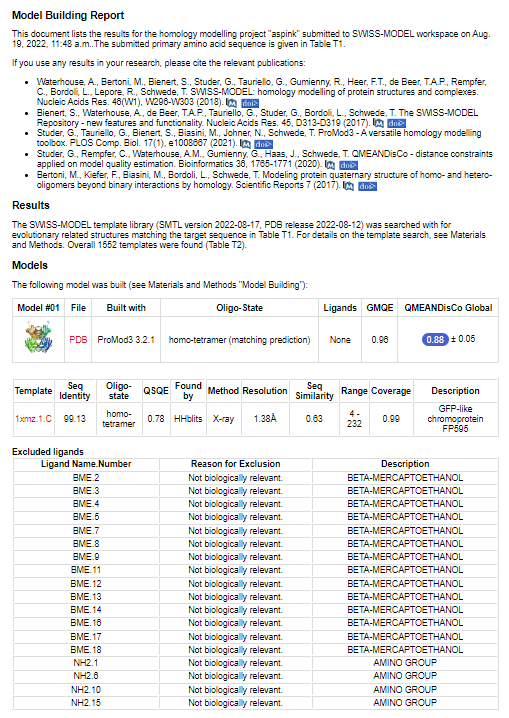
| + | With bioinformation analysis using Swiss Model, we predicted the structure of aspink protein (Figure 4). | ||
| + | [[File:aspink-fig4.png|500px|thumb|center|Figure 4. Predicted structural model of aspink protein]] | ||
| + | <br> | ||
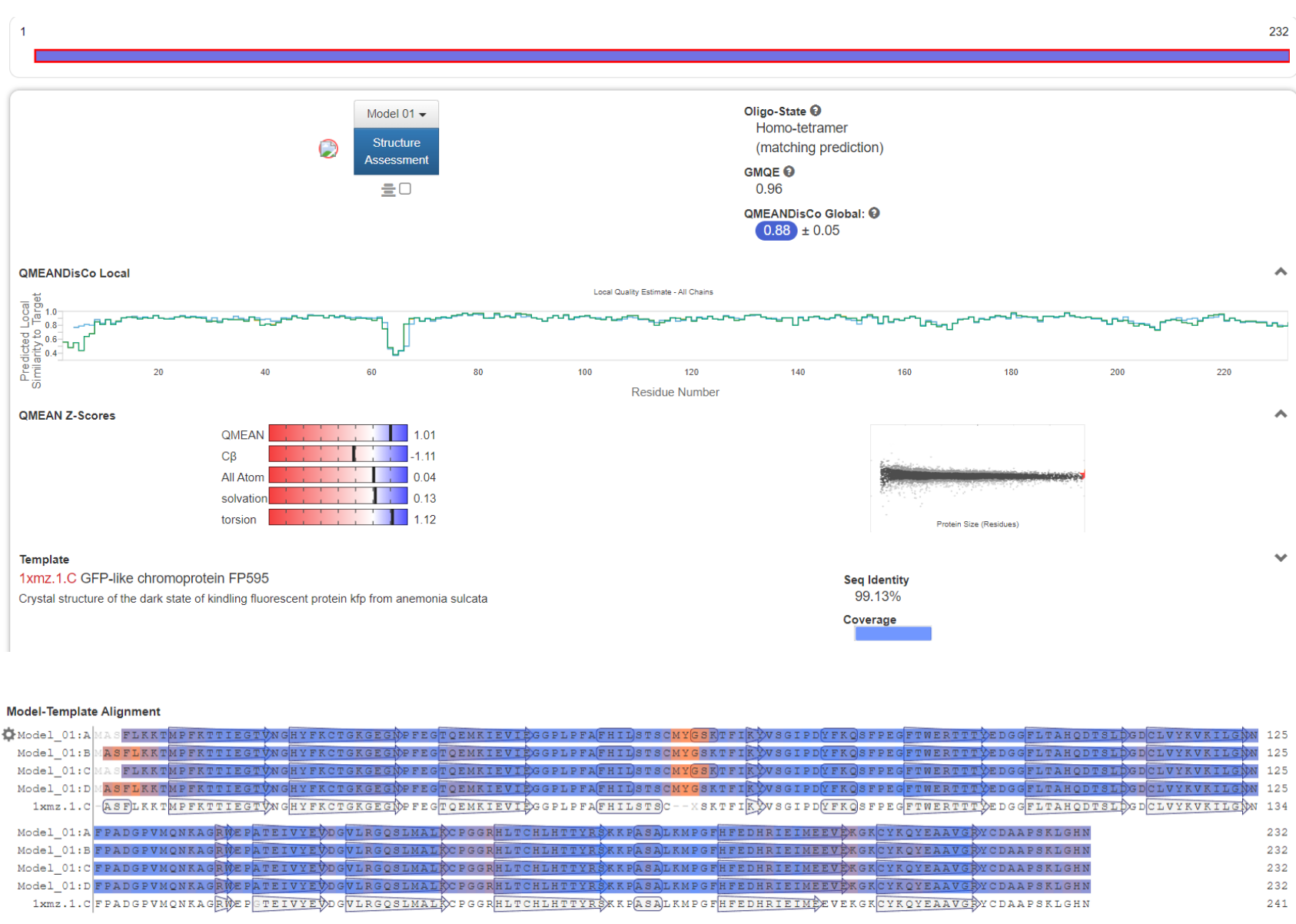
| + | Further the detailed characteristics of the protein were analyzed as shown in Figure 5. | ||
| + | <br> | ||
| + | [[File:aspink-fig5-1.png|500px|thumb|left|Figure 5-1. Bioinformatics analysis of aspink protein]] | ||
| + | [[File:aspink-fig5-2.png|300px|thumb|center|Figure 5-2. Bioinformatics analysis of aspink protein]] | ||
| + | [[File:aspink-fig5-3.png|300px|thumb|left|Figure 5-3. Bioinformatics analysis of aspink protein]] | ||
| + | [[File:aspink-fig5-4.png|300px|thumb|center|Figure 5-4 Bioinformatics analysis of aspink protein]] | ||
| + | [[File:aspink-fig5-5.png|300px|thumb|right|Figure 5-5 Bioinformatics analysis of aspink protein]] | ||
| + | <br> | ||
| + | <br> | ||
===4.Mass spectrometry of the relative molecular mass of aspink=== | ===4.Mass spectrometry of the relative molecular mass of aspink=== | ||
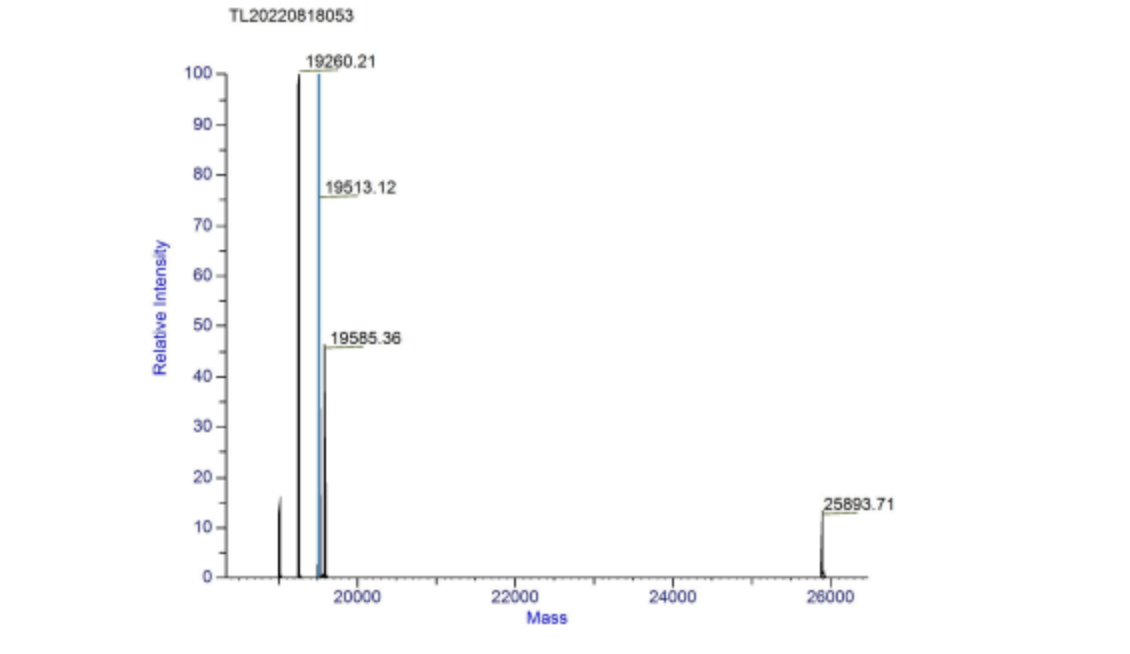
We used mass spectrometry to measure the relative molecular mass of aspink protein (Figure 6). The relative molecular mass measured was roughly 25.9 kDa, consistent with our prediction. The majority 19 kDa implicates that the expressed protein was not intact. | We used mass spectrometry to measure the relative molecular mass of aspink protein (Figure 6). The relative molecular mass measured was roughly 25.9 kDa, consistent with our prediction. The majority 19 kDa implicates that the expressed protein was not intact. | ||
| + | [[File:aspink-fig6.png|500px|thumb|center|Figure 6. Deconvolution spectrum of aspink protein]] | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Latest revision as of 04:03, 12 October 2022
asPink, pink chromoprotein
This chromoprotein from the coral Anemonia sulcata, asPink (also known as asCP or asFP595), naturally exhibits strong color when expressed. The protein has an absorption maximum at 572 nm giving it a pink/purple color visible to the naked eye. The strong color is readily observed in both LB or on agar plates after less than 24 hours of incubation. The protein asPink has significant sequence homologies with proteins in the GFP family.
Important: This part is not available in the registry yet, however, the same part is available from the registry with the standard RBS B0034: BBa_K1033927.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
This part is useful as a reporter.
Contribution
Group: Linkoping_Sweden iGEM 2019
Author: Andreas Holmqvist, Leo Juhlin and Emmanuel Berlin
Summary: We studied the expression in of E.coli BL21(DE3) containing CBD-asPink (BBa_K3182100) protein with a constitutive promotor to see if the protein could be used for a pink/white screening. We also tested the bindning capacity of the CBD-asPink protein to a micro cellulose bandage and then studied the release mechanism.
Documentation:
The expression system used contained the following parts:
- the BBa_B0034 Ribosome binding site
- the BBa_J23110 Constitutive promotor
Pink-white screening
To visualize the absorbance of AsPink on a petri dish, BL21 (DE3) colonies containing the pUC19-pCons-AsPink part (BBa_K3182100), which was used in the pink-white screening, were incubated for 16 hours in 37 °C. The pCons-AsPink construct was removed and (Magainin 2) and BBa_K3182104 (CHAP) was inserted into the vector. The pink colonies contain AsPink and indicate a religated pCons-AsPink. The white colonies indicate a successful ligation. Below (Figure 1) is a picture of the pink-white screening.

CBD-asPink bindning capacity
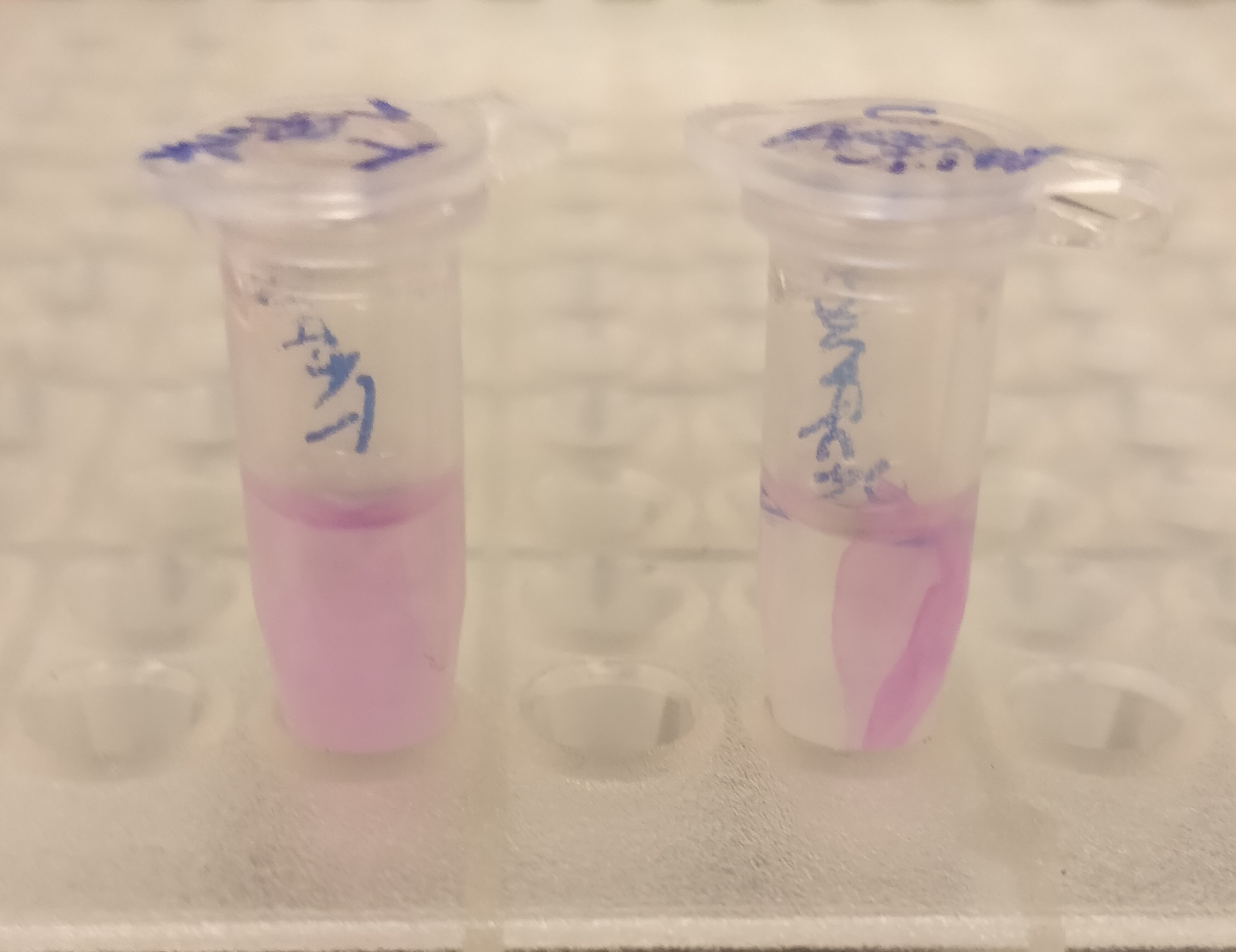
After the washes, human thrombin and cleavage buffer was added to the bandage with bound CBD-asPink to test the release mechanism of the fusion protein. The bandage was then incubated with the solution for 16 hours on an end to end rotator together with an negative control containing cellulose bandage and only cleavage buffer. After the incubation, the supernatant containing thrombin was pink while the negative control containing only cleavage buffer was transparent with a clear pink cellulose bandage (Figure 4). This indicates that the release mechanism work and the AsPink protein has successfully been released from the bandage into the supernatant.
asPink does not exist as CDS only but only as RBS-asPink (BBa_K1033927) and J23110-RBS-spisPink (BBa_K1033926).
iGEM2013 Uppsala: Expression of asPink in E. coli DH5alpha by promoter J23110 from medium copy plasmid pSB3K3 (left) or high copy plasmid pSB1K3 (right).
Source
The protein was first extracted and characterized by Lukyanov et. al. 2000 under the name asFP595 (GenBank: AAG02385.1). This version is codon optimized for E coli by Genscript.
References
[http://www.jbc.org/content/275/34/25879.short]Lukyanov, Konstantin A., et al. "Natural animal coloration can be determined by a nonfluorescent green fluorescent protein homolog." Journal of Biological Chemistry 275.34 (2000): 25879-25882.
[http://www.jbc.org/content/280/4/2401.short]Wilmann, Pascal G., et al. "Variations on the GFP Chromophore - A Polypeptide Fragmentation Within The Chromophore Revealed In The 2.1-Å Crystal Structure Of A Nonfluorescent Chromoprotein From Anemonia Sulcata." Journal of Biological Chemistry 280.4 (2005): 2401-2404.
Characterization from iGEM22_Worldshaper-Nanjing
Group: Worldshaper-Nanjing 2022iGEM
Author: Ziqi Huang,Yunzheng Tian
Summary: Characterization of the expression of aspink in E.coli strain BL21 transformed with pET-29a-aspink plasmid, the purification of aspink protein and the bioinformatic information of aspink protein.
We obtained the plasmid vector containing this part, pET-29a-aspink plasmid, from iGEM team iGEM13_Uppsala. We transformed this plasmid into E.coli strain BL21, and induced aspink expression. We extracted and purified the aspink protein by sonication, ultrafiltration, and Ni-affinity chromatography. With bioinformation analysis using Swiss Model, we predicted the structure of aspink protein and analyzed detailed characteristics of the protein. The relative molecular mass was measured by mass spectrometry.
Experiments Results
1.Induced expression of aspink in E. coli BL21 transformed with pET-29a aspink plasmid
The growth performance of the BL21 bacteria samples transformed with pET-29a-aspink plasmid was measured. Two samples with OD value around 0.6 were used as the control group and the induced group, respectively. IPTG was added to induce aspink expression (Figure 1). Protein was extracted and purified from the two groups by sonication, ultrafiltration, and Ni-affinity chromatography (Figure 2).
2.SDS-PAGE verification of aspink expression
SDS-PAGE experiment verified the successful expression of aspink protein induced in BL21 (Figure 3). The size of aspink is predicted around 26 kDa.
3.Aspink Bioinformation Analysis
With bioinformation analysis using Swiss Model, we predicted the structure of aspink protein (Figure 4).
Further the detailed characteristics of the protein were analyzed as shown in Figure 5.
4.Mass spectrometry of the relative molecular mass of aspink
We used mass spectrometry to measure the relative molecular mass of aspink protein (Figure 6). The relative molecular mass measured was roughly 25.9 kDa, consistent with our prediction. The majority 19 kDa implicates that the expressed protein was not intact.