Difference between revisions of "Featured Parts:Conjugation parts"
Smelissali (Talk | contribs) |
(→More Information/References) |
||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 10: | Line 10: | ||
==Background/About Conjugation== | ==Background/About Conjugation== | ||
| − | + | {{:Conjugation/Overview}} | |
| − | + | ||
| − | + | ==Suggested Uses for these BioBricks== | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | So what can I do with these BioBricks? | |
| − | + | *The advantages to these BioBricks is in their ability to separate out the OriTf gene, which is responsible for the physical movement of DNA from cell to cell, from the rest of the F-plasmid. | |
| − | So what can I do with these | + | |
| − | *The advantages to these | + | |
Thus it is possible to transfer engineered plasmids from cell to cell in the presence of either a wild type F-plasmid or the O-lambda F plasmid (F plasmid with the gene for OriTf knocked out), provided that the engineered plasmid contains the OriTf gene. | Thus it is possible to transfer engineered plasmids from cell to cell in the presence of either a wild type F-plasmid or the O-lambda F plasmid (F plasmid with the gene for OriTf knocked out), provided that the engineered plasmid contains the OriTf gene. | ||
| Line 30: | Line 22: | ||
===Technical=== | ===Technical=== | ||
''The Process of Conjugation'' itself is most often carried out in the lab by either of the two following processes | ''The Process of Conjugation'' itself is most often carried out in the lab by either of the two following processes | ||
| − | #Liquid Media: Recipient culture and donor culture are grown separately in liquid media overnight at | + | #Liquid Media: Recipient culture and donor culture are grown separately in liquid media overnight at 37 C. Add both cultures to the same sterile container. Allow a half hour to two hours for conjugation to occur. Do not disturb (ie. shake) the container--it will cause the pili to shear and thus prevent conjugation. (Note: this method is suitable for the F-type conjugal plasmid carriers, but not the R-type conjugal plasmid, whose pili are not as robust) |
#Solid Media: Recipient culture and donor cultures are grown separately overnight on solid agar plates. Mix cells together on the same sterile agar plate using an innoculating loop, streaking, etc. This method is suitable for all types of conjugative plasmids (F, R, etc). | #Solid Media: Recipient culture and donor cultures are grown separately overnight on solid agar plates. Mix cells together on the same sterile agar plate using an innoculating loop, streaking, etc. This method is suitable for all types of conjugative plasmids (F, R, etc). | ||
| − | #Official protocols from published journal articles are available [http:// | + | #Official protocols from published journal articles are available [http://2006.igem.org/BerkeleyConjugation here] |
===Previous Experiments=== | ===Previous Experiments=== | ||
| Line 43: | Line 35: | ||
==More Information/References== | ==More Information/References== | ||
| − | + | {{:Conjugation/References}} | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
Latest revision as of 02:55, 12 December 2008
Contents
The Parts
- BBa_J01002 "OriTf" - Origin of Transfer for the F-type conjugative plasmid
- BBa_J01003 "OriTr" - Origin of Transfer for the R-type conjugative plasmid
- BBa_J01000 "TraJf" - Initiates expression of mating characteristic in the F-type conjugative plasmid
- BBa_J01001 "TraJr" - Initiates mating characteristics in the R-type conjugative plasmid
- BBa_J01078 "R plasmid (x-OriT, x-TraJ)" - Modified R plasmid lacking the TraJr and OriTr genes
- BBa_J01077 "F plasmid (x-OriT, x-TraJ)" - Modified F plasmid lacking the TraJr and OriTr genes
Background/About Conjugation
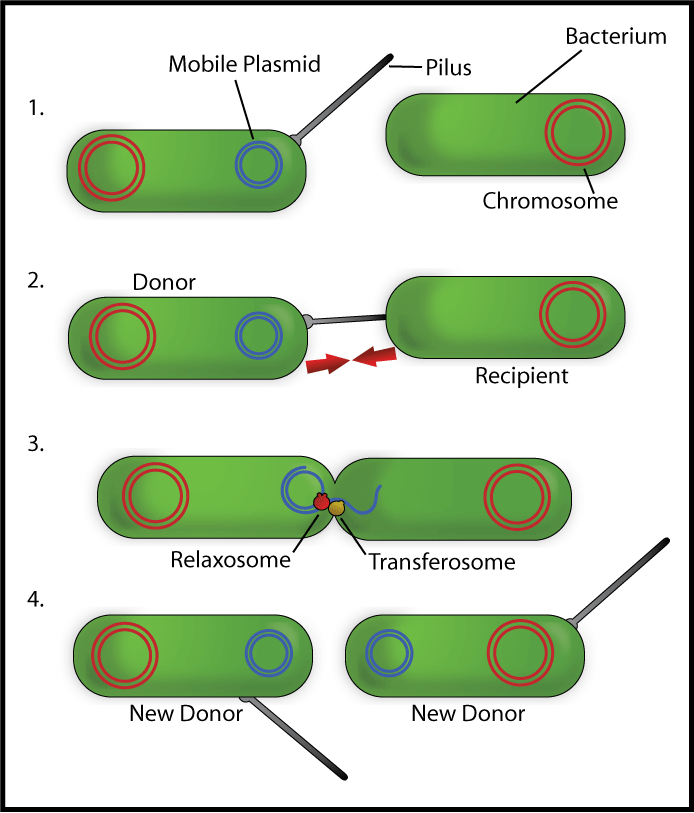
Bacterial conjugation is the transfer of genetic material between two bacterial cell via direct cell-to-cell contact. Although often incorrectly characterized as the bacterial equivalent of mating, in fact it is simply the transfer of genetic material from a donor cell to a recipient cell. Bacterial conjugation is also known as a "type IV secretion system".
This behaviour is characterized by the presence of a specialized plasmid (small, circular piece of transferrable DNA most often found within bacteria) known as a conjugative plasmid. The conjugative plasmid holds a distinct and specialized set of coding regions including the following
- OriT (Origin of Transfer): Unlike other plasmids, the conjugative plasmid has its own origin of transfer nic region. This is a specialized portion of the genome where one of the two strands of the circular plasmid DNA is cut to allow for rolling circle replication of the plasmid containing the OriT region into a recipient cell. During rolling circle replication, the cut end of one strand is inserted into the recipient cell while recipient polymerases construct the complementary strand back into a circular piece of DNA.
- TraJ (Transfer genes): The activation of this gene sets off a cascade of other plasmid genes (and thus corresponding protein expressions) which act in concert to form "mating" characteristics in the host bacterial cell. Such salient characteristics include:
- growth of a pilus--a long whip-like apparatus used to hook the two cells together
- fusion of the outer membranes to allow for transfer of genetic material
- formation of "surface exclusion proteins" which prevents the cell containing the conjugative plasmid from mating with other conjugative plasmids of its type
Two of the most well-studied and characterized types of conjugative plasmids are types "F" (from the F plasmid) and "R". The employed nomenclature for conjugative plasmid genes is the gene name first with the type afterwards. For example, the "TraJr" or "TraJR" gene denotes the "TraJ" gene of the "R" type conjugative plasmid.
Note it was previously believed that transfer of genetic material occurred via the pilus itself; now, most think that the transfer of genetic material occurs via a separate direct channel between the two cells.
Suggested Uses for these BioBricks
So what can I do with these BioBricks?
- The advantages to these BioBricks is in their ability to separate out the OriTf gene, which is responsible for the physical movement of DNA from cell to cell, from the rest of the F-plasmid.
Thus it is possible to transfer engineered plasmids from cell to cell in the presence of either a wild type F-plasmid or the O-lambda F plasmid (F plasmid with the gene for OriTf knocked out), provided that the engineered plasmid contains the OriTf gene.
Experimental and Technical
Technical
The Process of Conjugation itself is most often carried out in the lab by either of the two following processes
- Liquid Media: Recipient culture and donor culture are grown separately in liquid media overnight at 37 C. Add both cultures to the same sterile container. Allow a half hour to two hours for conjugation to occur. Do not disturb (ie. shake) the container--it will cause the pili to shear and thus prevent conjugation. (Note: this method is suitable for the F-type conjugal plasmid carriers, but not the R-type conjugal plasmid, whose pili are not as robust)
- Solid Media: Recipient culture and donor cultures are grown separately overnight on solid agar plates. Mix cells together on the same sterile agar plate using an innoculating loop, streaking, etc. This method is suitable for all types of conjugative plasmids (F, R, etc).
- Official protocols from published journal articles are available [http://2006.igem.org/BerkeleyConjugation here]
Previous Experiments
- Canonical Experiment: The first conjugation experiments were proven using antibiotic resistances. This is easily done through the following method.
- Take two populations of cells:
- one of which carries the F-type conjugal plasmid along with a select marker 1 or antibiotic resistance 1 (ie. RFP or Tet resistance) on the same F-type conjugal plasmid and
- one population which does not carry the F-type plasmid but does carry a different selection resistance (ie. Chloramphenicol resistance) within its own cellular genome.
- Conjugate the two populations (see above for some suggested techniques)
- The resulting population should carry both marker 1/antibiotic resistance 1 as well as antibiotic resistance 2.
- Take two populations of cells:
More Information/References
<biblio>
- Frost pmid=7915817
- Jaenecke pmid=8858584
- Lawley pmid=12855161
- Lessl pmid=8407818
- Miller pmid=2993231
- Martinez-Morales pmid=10559184
- Wilkins pmid=12220405
</biblio>
- [http://en.wikipedia.org/wiki/Bacterial_conjugation Wikipedia entry on Bacterial Conjugation]
- 2005 UC Berkeley iGEM team [http://2006.igem.org/Berkeley_2005 project] and [http://2006.igem.org/Berkeley_Protocols protocols]
