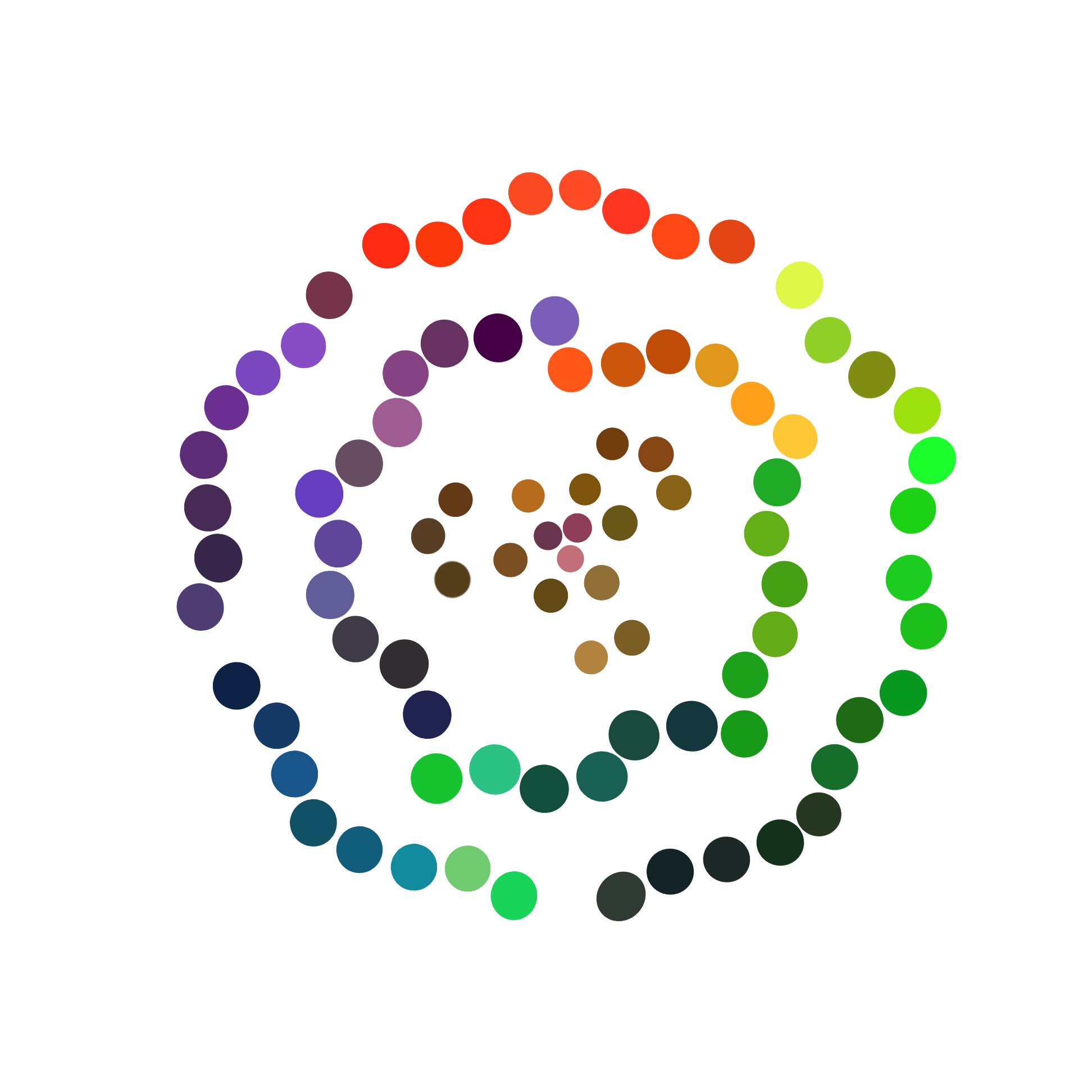Difference between revisions of "Part:BBa K2598038"
(→2019 Team XJTU-CHINA improvement) |
|||
| (5 intermediate revisions by 2 users not shown) | |||
| Line 45: | Line 45: | ||
===Overview=== | ===Overview=== | ||
| − | This year team XJTU-CHINA | + | This year team XJTU-CHINA aim to improve a well-characterized red-light-activated sensor which is part of the GRB system of 2018 team UCAS-China(Part:BBa_K2598038). This part contains red-light sensor cph8(BBa_I15010) and repressor CI (BBa_C0051) under its regulation. Base on this red-light-activated sensor, we add a CI regulated promoter - Pλ to this part and enable it to realize the function of two-phases switch (BBa_K3052030). Besides, we also added two fluorescent proteins - superfolder GFP (BBa_K3052041) and mRFP (BBa_K3052042) under the control of pomp (BBa_R0082) and Pλ(BBa_K1145005) respectively to report the expression level of each phase. Theoretically, RFP will expresses under red light and GFP expresses in the absence of red light. |
| + | |||
| + | <div>[[File:T--XJTU-CHINA--JF3.png |700px|thumb|center|<b>Figure 1:</b>The relationship between the red fluorescent intensity of E. coli under light of 620-630nm wavelength and illumination intensity]]</div> | ||
| + | |||
| + | |||
| + | UCAS-2018 tested the change of fluorescence intensity with the change of illumination intensity. | ||
| + | When there is no light, fluorescence is not expressed, but when there is light, fluorescence starts to be expressed, and with the increase of time and the increase of light intensity, fluorescence intensity increases, which is shown as a one-way switch. | ||
| + | Cite:http://2018.igem.org/Team:UCAS-China/Demonstrate | ||
<div>[[File:T--XJTU-CHINA--CIRCUIT.png |700px|thumb|center|<b>Figure 1:</b>bidirectional light-responsive switch designed by 2019 XJTU-CHINA]]</div> | <div>[[File:T--XJTU-CHINA--CIRCUIT.png |700px|thumb|center|<b>Figure 1:</b>bidirectional light-responsive switch designed by 2019 XJTU-CHINA]]</div> | ||
| + | |||
| + | Reference | ||
| + | [1]Schmidl, S. R., Sheth, R. U., Wu, A., & Tabor, J. J. (2014). | ||
| + | Refactoring and optimization of light-switchable Escherichia coli two-component systems. | ||
| + | ACS synthetic biology, 3(11), 820-831. | ||
| + | |||
| + | [2]http://2018.igem.org/Team:UCAS-China/Design | ||
===Characterization=== | ===Characterization=== | ||
| Line 66: | Line 80: | ||
To quantitatively test the efficiency of this light switch, we decided to cultivate the cells in dark environment first and then trigger the switch with intense red light. When they had been incubated for 30 hours without red light, we start to put intense red light around the medium. During the experiment, we measured the GFP and RFP intensity of the sample every 2 hours. | To quantitatively test the efficiency of this light switch, we decided to cultivate the cells in dark environment first and then trigger the switch with intense red light. When they had been incubated for 30 hours without red light, we start to put intense red light around the medium. During the experiment, we measured the GFP and RFP intensity of the sample every 2 hours. | ||
| − | <div>[[File:T--XJTU-CHINA--QUA.png |700px|thumb|center|<b>Figure 4:</b> Quantitative result of GFP and RFP fluorescent intensity of the light-controlling switch.(The blue dotted line represents the time point of starting red light treatment)]]</div> | + | <div>[[File:T--XJTU-CHINA--QUA.png |700px|thumb|center|<b>Figure 4:</b> Quantitative result of GFP and RFP fluorescent intensity of the light-controlling switch.(The blue dotted line represents the time point of starting red light treatment and heat at 42℃)]]</div> |
| − | From | + | From above figure we can see that the GFP intensity increased gradually while RFP intensity maintained to be at a low basic level without red light. But after red light treatment, GFP intensity dropped sharply to a low and stable value in 4 hours, and slow increase of RFP intensity was obviously observed. The results clearly indicates the effectiveness of this bidirection switch and the fast response time to the red-light stimulation. |
All in all, the results quantitively and quantitatively demonstrated the light controlled switch is functional. Compared to the previous part which achieved the monodirectional regulations (BBa_K2598038 of UCAS_China 2018) (http://2018.igem.org/Team:UCAS-China), our improved version can efficiently achieve bidirectional regulation of two genes, leading to more flexible and selective regulations. BBa_K3052030 in our project is the proof of concept of this bidirectional switch, and the fluorescent proteins will be alternated with our selected linalool synthase and limonene synthase which have also been proved to be functional, to achieve the selective production of linalool and limonene. | All in all, the results quantitively and quantitatively demonstrated the light controlled switch is functional. Compared to the previous part which achieved the monodirectional regulations (BBa_K2598038 of UCAS_China 2018) (http://2018.igem.org/Team:UCAS-China), our improved version can efficiently achieve bidirectional regulation of two genes, leading to more flexible and selective regulations. BBa_K3052030 in our project is the proof of concept of this bidirectional switch, and the fluorescent proteins will be alternated with our selected linalool synthase and limonene synthase which have also been proved to be functional, to achieve the selective production of linalool and limonene. | ||
Latest revision as of 03:13, 22 October 2019
cph8+CI
This part contains red-light sensor cph8(BBa_I15010) and repressor CI(BBa_C0051) under its regulation. Together they can serve as awell-characterized red-light-activated sensor. It is part of the GRB system, see more information from RGB System
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 2298
Illegal XhoI site found at 405 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Characterization
Figure 1 shows the relationship between the wavelength of light exposed on liquid medium and the intensity of BFP, GFP and RFP E. coli expressed from left figure to right figure respectively. We got the data through flow cytometer and analyzed it to get the figure. The y-axis is the number of cells, and the x-axis is fluorescence intensity. And every color is E. coli that grows for 8 hours under the light of the corresponding wavelength. We can see E. coli has the highest blue fluorescence expression under blue light from the left graph. And We can also see E. coli has the highest green and red fluorescence expression under green light and right light from the middle and right graph respectively. So this figure proves that our system and our parts can work well.
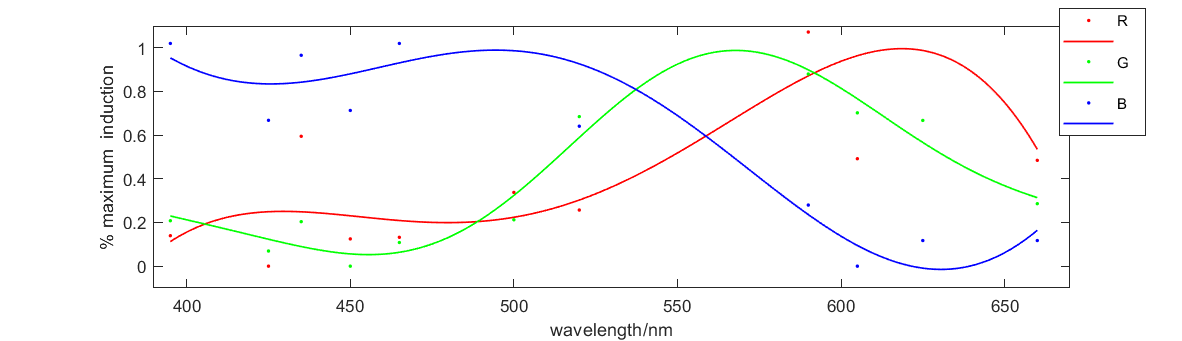
Figure 2 shows the relationship between fluorescence intensity and excitation wavelength. The x-axis is wavelength of 10h illumination. The solid medium gradually emerged and the y-axis is RGB figure of fluorescence in illuminated solid medium. This curve illustrates how our system responses to different excitation wavelength, which perfectly meets our expectation. So this figure proves that our system and our parts can work well.
Figure 3 shows colors we got from the solid medium exposed under light, in which E. coli producing fluorescent protein grows. When E .coli producing fluorescent protein are exposed under uniform light of single wavelength, the solid medium gradually emerged corresponding colors. And using color picker, we get many pure colors with predominant continuity.
We explored the relationship between fluorescent intensity and illumination intensity, which affects the shade of the color.
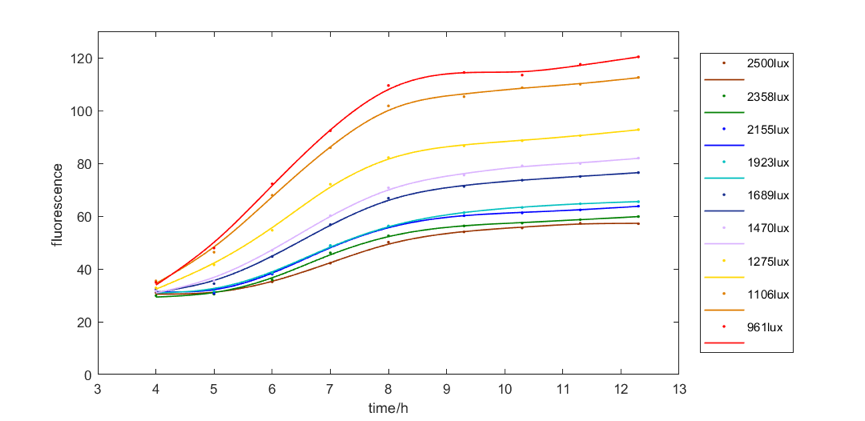
Figure 4 shows the red fluorescent intensity of E. coli under light of 620-630nm wavelength with different illumination intensity. We found when illuminated under around 961lux light, we can get the most red fluorescence. We also explore the relationship between green and blue fluorescent intensity of E. coli under light of 515-530nm wavelength and 460-470nm wavelength respectively and illumination intensity. The results are similar, that is, moderate intensity of light is most favorable for E. coli to express fluorescence.
2019 Team XJTU-CHINA improvement
Overview
This year team XJTU-CHINA aim to improve a well-characterized red-light-activated sensor which is part of the GRB system of 2018 team UCAS-China(Part:BBa_K2598038). This part contains red-light sensor cph8(BBa_I15010) and repressor CI (BBa_C0051) under its regulation. Base on this red-light-activated sensor, we add a CI regulated promoter - Pλ to this part and enable it to realize the function of two-phases switch (BBa_K3052030). Besides, we also added two fluorescent proteins - superfolder GFP (BBa_K3052041) and mRFP (BBa_K3052042) under the control of pomp (BBa_R0082) and Pλ(BBa_K1145005) respectively to report the expression level of each phase. Theoretically, RFP will expresses under red light and GFP expresses in the absence of red light.
UCAS-2018 tested the change of fluorescence intensity with the change of illumination intensity.
When there is no light, fluorescence is not expressed, but when there is light, fluorescence starts to be expressed, and with the increase of time and the increase of light intensity, fluorescence intensity increases, which is shown as a one-way switch.
Cite:http://2018.igem.org/Team:UCAS-China/Demonstrate
Reference [1]Schmidl, S. R., Sheth, R. U., Wu, A., & Tabor, J. J. (2014). Refactoring and optimization of light-switchable Escherichia coli two-component systems. ACS synthetic biology, 3(11), 820-831.
[2]http://2018.igem.org/Team:UCAS-China/Design
Characterization
The light-fluorescent circuits (BBa_K3052030) were constructed by two-step Golden Gate Assembly and were confirmed by colony PCR. The length of this fragment is 1467 bp and the following results showed the successful construction of plasmid.
In order to validate the effectiveness of this switch, we qualitatively measure the fluorescent of this circuit at different time. Sample of DH5α E. coli transformed with BBa_K3052030 is incubated for 24 hours without red light and then continue incubating 24 hours with red light. We photographed both the sample of control group (DH5α E.coli wildtype) and experimental group(E.coli with light-fluorescent circuit ) by a fluorescence microscope at 24 hours (24 hours without light treatment) and 48 hours (24 h without light treatment and 24 h with red light treatment). The figure indicated that the bidirectional switch was functional to switch from GFP to RFP.

Furthermore, we used COFOCAL to sensitively detect GFP and RFP while switch is shifting. After the sample above had been incubated for 24 hours without light treatment, it was treated with intense red light (wavelength=640nm). After incubating for another 3 hours with red light treatment, we took out some of the sample and perform CONFOCAL imaging. The result shows that the RFP was appearing with red light treatment, which indicates this switch can be effectively triggered by red light.
To quantitatively test the efficiency of this light switch, we decided to cultivate the cells in dark environment first and then trigger the switch with intense red light. When they had been incubated for 30 hours without red light, we start to put intense red light around the medium. During the experiment, we measured the GFP and RFP intensity of the sample every 2 hours.
From above figure we can see that the GFP intensity increased gradually while RFP intensity maintained to be at a low basic level without red light. But after red light treatment, GFP intensity dropped sharply to a low and stable value in 4 hours, and slow increase of RFP intensity was obviously observed. The results clearly indicates the effectiveness of this bidirection switch and the fast response time to the red-light stimulation.
All in all, the results quantitively and quantitatively demonstrated the light controlled switch is functional. Compared to the previous part which achieved the monodirectional regulations (BBa_K2598038 of UCAS_China 2018) (http://2018.igem.org/Team:UCAS-China), our improved version can efficiently achieve bidirectional regulation of two genes, leading to more flexible and selective regulations. BBa_K3052030 in our project is the proof of concept of this bidirectional switch, and the fluorescent proteins will be alternated with our selected linalool synthase and limonene synthase which have also been proved to be functional, to achieve the selective production of linalool and limonene.