Difference between revisions of "Part:BBa K3037006"
(→Measurement of fluorescence: Performed in the composite BioBrick BBa_K3037005) |
|||
| (8 intermediate revisions by 2 users not shown) | |||
| Line 4: | Line 4: | ||
|- | |- | ||
|'''Function''' | |'''Function''' | ||
| − | |Reporter | + | |Expression, Reporter |
|- | |- | ||
|'''Use in''' | |'''Use in''' | ||
| Line 13: | Line 13: | ||
|- | |- | ||
|'''Backbone''' | |'''Backbone''' | ||
| − | |pSB1C3<br> | + | |[https://parts.igem.org/Help:2019_DNA_Distribution pSB1C3<br>] |
| + | |- | ||
| + | |'''Experimental Backbone''' | ||
| + | |[https://parts.igem.org/Part:BBa_K3037000 pOCC97<br>] | ||
|- | |- | ||
|'''Submitted by''' | |'''Submitted by''' | ||
| Line 27: | Line 30: | ||
Made by the TU Dresden 2019 adapted to the RFC 25 standard [https://2019.igem.org/Team:TU_Dresden/Parts (more information).] | Made by the TU Dresden 2019 adapted to the RFC 25 standard [https://2019.igem.org/Team:TU_Dresden/Parts (more information).] | ||
| + | === Biology === | ||
| + | Traditionally isolated from the jellyfish Aequorea victoria, the green fluorescent protein (GFP) | ||
| + | has become an important marker for gene expression [1]. However, due to the relatively lower | ||
| + | sensitivity of wild-type GFP compared to standard reporter proteins, many different mutants of | ||
| + | GFP have been engineered. | ||
| + | Thereby, mutations introduced in chromophore of GFP significantly improved its detection and | ||
| + | made the protein 35 times brighter than wild-type GFP. The modified fluorescent protein, also | ||
| + | known as enhanced GFP (eGFP), appeared to be more sensitive compared to initial detection | ||
| + | results [2]. | ||
| + | == Characterization == | ||
| + | ==== Measurement of fluorescence: Performed in the composite BioBrick [https://parts.igem.org/Part:BBa_K3037005 BBa_K3037005] ==== | ||
| + | |||
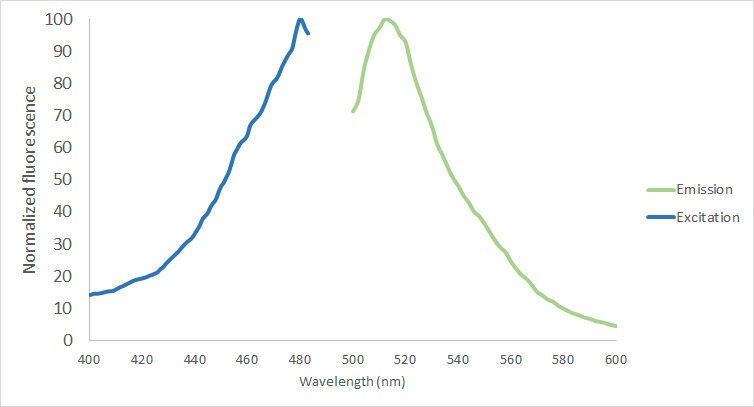
| + | The protein functionality of eGFP in the fusion protein was analyzed in the protein purified using the MBP-tag. First of all, the excitation and emission spectrum were measured with Tecan Plate Reader (Bandwith 20 nm) (Figure 5). The excitation maxima was screened from 400 to 490, being the final excitation maxima 480 nm. The emission maxima was measured from 500 to 600 nm with a final result of 512 nm. | ||
| + | |||
| + | |||
| + | [[File:T--TU_Dresden--Fluorescence_BBa_K3037005.png|center|400px|thumb|left|Figure 5: Fluorescense spectrum measurement. Excitation maxima 480 nm. Emission maxima 512 nm.]] | ||
== Sequence == | == Sequence == | ||
| Line 35: | Line 54: | ||
<partinfo>BBa_K3037006 SequenceAndFeatures</partinfo> | <partinfo>BBa_K3037006 SequenceAndFeatures</partinfo> | ||
| + | == References == | ||
| + | |||
| + | [1] Stepanenko OV, Verkhusha VV, Kuznetsova IM, Uversky VN, Turoverov KK (Aug 2008). | ||
| + | "Fluorescent proteins as biomarkers and biosensors: throwing color lights on molecular and | ||
| + | cellular processes". Current Protein & Peptide Science. 9 (4): 338–69 | ||
| + | |||
| + | [2] Zhang, G., Gurtu, V., & Kain, S. R. (1996). An Enhanced Green Fluorescent Protein Allows | ||
| + | Sensitive Detection of Gene Transfer in Mammalian Cells. Biochemical and Biophysical | ||
| + | Research Communications, 227(3), 707–711. | ||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
Latest revision as of 02:09, 22 October 2019
eGFP
| eGFP | |
|---|---|
| Function | Expression, Reporter |
| Use in | Escherichia coli |
| RFC standard | Freiburg RFC25 standard |
| Backbone | pSB1C3 |
| Experimental Backbone | pOCC97 |
| Submitted by | Team: TU_Dresden 2019 |
Contents
Overview
Made by the TU Dresden 2019 adapted to the RFC 25 standard (more information).
Biology
Traditionally isolated from the jellyfish Aequorea victoria, the green fluorescent protein (GFP) has become an important marker for gene expression [1]. However, due to the relatively lower sensitivity of wild-type GFP compared to standard reporter proteins, many different mutants of GFP have been engineered. Thereby, mutations introduced in chromophore of GFP significantly improved its detection and made the protein 35 times brighter than wild-type GFP. The modified fluorescent protein, also known as enhanced GFP (eGFP), appeared to be more sensitive compared to initial detection results [2].
Characterization
Measurement of fluorescence: Performed in the composite BioBrick BBa_K3037005
The protein functionality of eGFP in the fusion protein was analyzed in the protein purified using the MBP-tag. First of all, the excitation and emission spectrum were measured with Tecan Plate Reader (Bandwith 20 nm) (Figure 5). The excitation maxima was screened from 400 to 490, being the final excitation maxima 480 nm. The emission maxima was measured from 500 to 600 nm with a final result of 512 nm.
Sequence
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
References
[1] Stepanenko OV, Verkhusha VV, Kuznetsova IM, Uversky VN, Turoverov KK (Aug 2008). "Fluorescent proteins as biomarkers and biosensors: throwing color lights on molecular and cellular processes". Current Protein & Peptide Science. 9 (4): 338–69
[2] Zhang, G., Gurtu, V., & Kain, S. R. (1996). An Enhanced Green Fluorescent Protein Allows Sensitive Detection of Gene Transfer in Mammalian Cells. Biochemical and Biophysical Research Communications, 227(3), 707–711.