Difference between revisions of "Part:BBa K3037003"
(→Characterization: fixxxxed) |
|||
| Line 1: | Line 1: | ||
| + | <partinfo>BBa_K3037003 short</partinfo> | ||
| + | {| style="color:black" cellpadding="6" cellspacing="1" border="2" align="right" | ||
| + | ! colspan="2" style="background:#FFBF00;"|Fusion protein | ||
| + | |- | ||
| + | |'''Function''' | ||
| + | |Colour detection of specific DNA sequences | ||
| + | |- | ||
| + | |'''Use in''' | ||
| + | |<span style="font-style: italic;">Escherichia coli</span> | ||
| + | |- | ||
| + | |'''RFC standard''' | ||
| + | |[[Help:Assembly_standard_25|Freiburg RFC25 standard]] | ||
| + | |- | ||
| + | |'''Backbone''' | ||
| + | |[https://parts.igem.org/Help:2019_DNA_Distribution pSB1C3<br>] | ||
| + | |- | ||
| + | |'''Experimental Backbone''' | ||
| + | |[https://parts.igem.org/Part:BBa_K3037000 pOCC97<br>] | ||
| + | |- | ||
| + | |'''Submitted by''' | ||
| + | |Team: [https://2019.igem.org/Team:TU_Dresden TU_Dresden 2019] | ||
| + | |} | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | == Overview == | ||
| + | |||
| + | |||
| + | The [https://2019.igem.org/Team:TU_Dresden TU Dresden 2019] team has designed this BioBrick in order to allow for the quick detection of specific DNA sequences of interest [https://2019.igem.org/Team:TU_Dresden/Parts (more information).] | ||
| + | |||
| + | The Full Construct as it is shown in Figure 1 below, contains five individual parts each of which have been optimized for it. | ||
| + | The MBP and Strep-tag allow purification via amylose resin and Strep-columns, respectively. | ||
| + | Additionally, the MBP enhances the expression of dCas9-fusion proteins, and the linker aids for the folding process. | ||
| + | The dCas9 identifies the sequence of interest (with the help of an added guideRNA), and the HRP provides with an easily detectable amplified color readout. | ||
| + | The single constructs were cloned into pSB1C3 and pSB1A3, and finally, the full construct was inserted into the [https://parts.igem.org/Part:BBa_K3037000 BBa_K3037000] vector for expression and characterization in <span style="font-style: italic;">Escherichia coli (E. coli)</span>. | ||
| + | |||
| + | The weight of the fusion protein was calculated in accordance to the number of base pairs. The sequence of our Full Construct is 924 bp/3 = 308 amino acids long, each amino acid weights on average 110 Dalton [1], which results in the final weight of approximately 230 kDa. | ||
| + | |||
| + | [[File:Fullconstruct.png|center|400px|thumb|none|Figure 1: Visualization of the full construct with its single parts.]] | ||
| + | |||
| + | == Biology == | ||
| + | |||
| + | For more information regarding the biology, design and function of each of the basic parts, please check the individual registry pages: | ||
| + | |||
| + | * MBP: [https://parts.igem.org/Part:BBa_K3037001 BBa_K3037001] | ||
| + | * dCas9: [https://parts.igem.org/Part:BBa_K3037002 BBa_K3037002] | ||
| + | * Linker: [https://parts.igem.org/Part:BBa_K3037004 BBa_K3037004] | ||
| + | * HRP: [https://parts.igem.org/Part:BBa_K3037007 BBa_K3037007] | ||
| + | * Strep-tag: [https://parts.igem.org/Part:BBa_K823038 BBa_K823038] | ||
| + | |||
| + | |||
| + | The expected parameters of the new protein (determined with ExPASy ProtParam tool) were determined to be: | ||
| + | |||
| + | * Extinction coefficient: 205750 L/(mol*cm) | ||
| + | * Estimated half-life > 30 hours in mammalian cells, >20 hours in yeast and >10 hours in <span style="font-style: italic;">E. coli</span> | ||
| + | |||
== Characterization == | == Characterization == | ||
| Line 5: | Line 64: | ||
We performed the following characterization experiments: | We performed the following characterization experiments: | ||
| − | <b>1)</b> Expression of | + | <b>1)</b> Expression of our Full Construct (FC) in pOCC97 [https://parts.igem.org/Part:BBa_K3037000 (BBa_K3037000):] and testing the growth of <i>E.coli</i>. |
| − | <b>2)</b> SDS-PAGEs | + | <b>2)</b> SDS-PAGEs showing the expression over time |
<b>3)</b> Image analysis of the expression in the SDS-PAGEs with ImageJ | <b>3)</b> Image analysis of the expression in the SDS-PAGEs with ImageJ | ||
| − | <b>4)</b> Characterization of the single parts of the | + | <b>4)</b> Characterization of the single parts of the full construct |
=== Experiments in Detail === | === Experiments in Detail === | ||
| − | ==== 1) Expression of | + | ==== 1) Expression of our Full Construct (FC) in pOCC97 [https://parts.igem.org/Part:BBa_K3037000 (BBa_K3037000):] and testing the growth of <i>E.coli</i>. ==== |
| − | + | The full construct, once all the single parts were fused together, was cloned into our expression plasmid K3037000 (p0CC97). The correct insertion of our full construct into the plasmid was proven via restriction digest followed by agarose gel electrophoresis. For that, we performed a triple digest with PmlI, X and P and got several positive clones. On the right, the simulation of the digest in SnapGene is shown (Figure 1). | |
| − | [[File:FC_digestion_gel_final.png|center|400px|thumb|none|Figure 1: Multiple | + | [[File:FC_digestion_gel_final.png|center|400px|thumb|none|Figure 1: Multiple full construct clones digested with PmlI, XbaI and Pst-1. On the right, the simulation in SnapGene is shown. The positive clones are marked with red crosses.]] |
| − | Furthermore, it was proven that the <span style="font-style: italic;">E. coli</span> could grow normally after the induction of the fusion protein. For | + | Furthermore, it was proven that the <span style="font-style: italic;">E. coli</span> could grow normally after the induction of the fusion protein. For that, the development of the bacteria cultures was monitored by measuring the OD at 600 nm during different time points before and after induction with 1 mM IPTG (Figure 2). |
| − | As shown in | + | As shown in the curve, the growth of the bacteria is not affected by the expression of the protein. Important to note here is that the expression of the full construct was performed in two slightly different pOCC97 plasmids, that differ on their Ribosome Binding Site (RBS). Herein after they are going to be referred to as optimized and not optimized (read the registry page [https://parts.igem.org/Part:BBa_K3037000 BBa_K3037000] to for more details regarding the difference between these two plasmids). |
[[File:T--TU_Dresden--Comparison_optimization_growing_curve_BBa_K3037000.png|center|400px|thumb|none|Figure 2: Comparison of the growth curve compared before and after optimization]] | [[File:T--TU_Dresden--Comparison_optimization_growing_curve_BBa_K3037000.png|center|400px|thumb|none|Figure 2: Comparison of the growth curve compared before and after optimization]] | ||
| − | To go further, the expression of the | + | To go further, the expression of the full construct in pOCC97 at different temperatures was studied. For that, the optimized and not optimized pOOC97 were compared (Figure 3). |
[[File:Growingcurves.png|center|700px|thumb|none|Figure 3: Comparison of the growth curves of optimized and not optimized pOCC97.]] | [[File:Growingcurves.png|center|700px|thumb|none|Figure 3: Comparison of the growth curves of optimized and not optimized pOCC97.]] | ||
| − | ==== 2) SDS-PAGEs | + | ==== 2) SDS-PAGEs showing the expression assay over time ==== |
| − | After proving that the final construct was well inserted in our plasmid, | + | After proving that the final construct was well inserted in our plasmid, the full construct was expressed overnight. The first expression was performed at 37°C for seven hours, induced with 1 mM IPTG. The result is shown below (Figure 4): |
| − | [[File:FC_expression.png|center|400px|thumb|none|Figure 4: SDS-page showing the expression of the | + | [[File:FC_expression.png|center|400px|thumb|none|Figure 4: SDS-page showing the expression of the full construct in pOCC97 after induction with 1 mM IPTG. Different type points show the increased expression of the full construct (marked with black arrow).]] |
| − | + | The same experiment was repeated several times at different temperatures and IPTG concentrations in both, the optimized and not optimized pOCC97 to compare the best expression conditions. The results are shown in the Figures below. | |
| − | |||
| − | [[File:T--TU_Dresden--Expression_FullConstruct_notoptimized_18_BBa_K3037000.png|center|400px|thumb|none|Figure 5: Expression of the Full Construct in not optimized pOCC97 at 18ºC | + | <b>Expression of Full Construct in pOCC97 not optimized at 18ºC and different IPTG concentrations</b> |
| + | |||
| + | [[File:T--TU_Dresden--Expression_FullConstruct_notoptimized_18_BBa_K3037000.png|center|400px|thumb|none|Figure 5: Expression of the Full Construct in not optimized pOCC97 at 18ºC]] | ||
| + | |||
<b>Expression of Full Construct in pOCC97 not optimized at 37ºC and different IPTG concentrations</b> | <b>Expression of Full Construct in pOCC97 not optimized at 37ºC and different IPTG concentrations</b> | ||
| − | [[File:T--TU_Dresden--Expression_FullConstruct_notoptimized_37.2_BBa_K3037000.png|center|400px|thumb|none|Figure 6: Expression of the Full Construct in not optimized pOCC97 at 37ºC | + | [[File:T--TU_Dresden--Expression_FullConstruct_notoptimized_37.2_BBa_K3037000.png|center|400px|thumb|none|Figure 6: Expression of the Full Construct in not optimized pOCC97 at 37ºC]] |
<b>Expression of the Full Construct in pOCC97 optimized at different temperatures and IPTG concentrations</b> | <b>Expression of the Full Construct in pOCC97 optimized at different temperatures and IPTG concentrations</b> | ||
| − | [[File:T--TU_Dresden--Expression_FullConstruct_optimized_BBa_K3037000.png|center|400px|thumb|none|Figure 7: Expression of the Full Construct in optimized pOCC97 at different temperatures and IPTG concentrations | + | [[File:T--TU_Dresden--Expression_FullConstruct_optimized_BBa_K3037000.png|center|400px|thumb|none|Figure 7: Expression of the Full Construct in optimized pOCC97 at different temperatures and IPTG concentrations]] |
==== 3) Image analysis of the expression in the SDS-PAGEs with ImageJ ==== | ==== 3) Image analysis of the expression in the SDS-PAGEs with ImageJ ==== | ||
| − | The previously shown SDS- | + | The previously shown SDS-pages were then further analysed by using the software ImageJ to correct for loading differences and be able to draw conclusions about the best conditions to express the Full Construct in pOCC97. |
<b>Temperature and IPTG induction dependence of the optimized pOCC97</b> | <b>Temperature and IPTG induction dependence of the optimized pOCC97</b> | ||
[[File:Clone7_dependences.png|center|600px|thumb|none|Figure 8: Expression of the Full Construct in optimized pOCC97 under different conditions.]] | [[File:Clone7_dependences.png|center|600px|thumb|none|Figure 8: Expression of the Full Construct in optimized pOCC97 under different conditions.]] | ||
| − | |||
| − | <b>Temperature and IPTG induction dependence of the not optimized | + | <b>Temperature and IPTG induction dependence of the not optimized POCC97</b> |
[[File:Clone11_dependences.png|center|600px|thumb|none|Figure 9: Expression of the Full Construct in not optimized pOCC97 under different conditions.]] | [[File:Clone11_dependences.png|center|600px|thumb|none|Figure 9: Expression of the Full Construct in not optimized pOCC97 under different conditions.]] | ||
| − | + | <b>Comparison between optimized and not optimized POCC97</b> | |
| + | [[File:Comparison_both.png|center|600px|thumb|none|Figure 10: Comparison between the expression of optimized and not optimized POCC97.]] | ||
| − | <b> | + | <b>Conclusion</b> |
| − | + | ||
| − | Based on | + | Based on this analysis it can be concluded that the optimal conditions for the expression of [https://parts.igem.org/Part:BBa_K3037003 BBa_3037003] in POCC97 are 18ºC and 0.5 mM IPTG. The expression seems to be more stable over time for the optimized plasmid than for the non-optimized. |
| − | ==== 4) Characterization of the single parts of the | + | ==== 4) Characterization of the single parts of the full construct ==== |
===== a) Purification via MBP ===== | ===== a) Purification via MBP ===== | ||
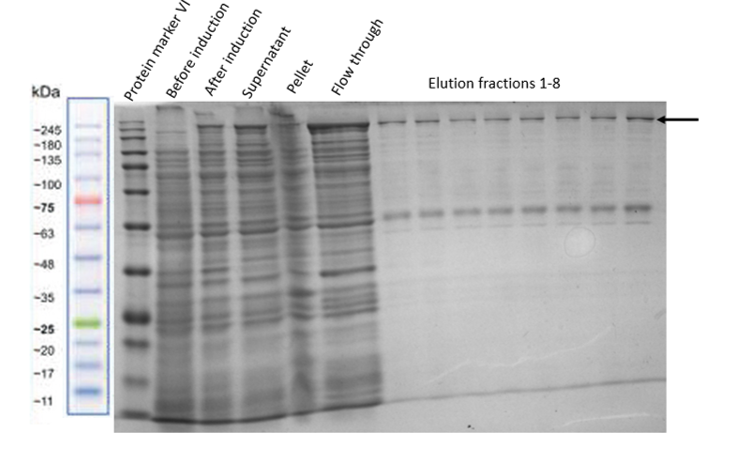
| − | After | + | After proving that the Full Construct is expressed properly in our plasmid and improved its expression conditions, it was purified by using Amylose Resin to bind its MBP site. To test for the correct functioning of each single part of the fusion protein we performed different experiments. For that, two different protocols were used. On the one hand, an Amylose Resin column was used and on the other hand a batch binding solution was prepared. Better results were obtained with the latter one and therefore, in which the resin was pipetted into a falcon and incubated with the cell lysate for 1.5 hours on a rotator at 4°C (Figure 11). |
| − | [[File:MBP_batchbinding.png|center|400px|thumb|none|Figure 11: | + | [[File:MBP_batchbinding.png|center|400px|thumb|none|Figure 11: Expression of the Full Construct in optimized pOCC97 and purification using batch binding solution]] |
===== b) Activity assay of HRP ===== | ===== b) Activity assay of HRP ===== | ||
| − | + | Investigating the activity of HRP in the Full Construct was done in a dynamic assay. The absorbance at 650 nm was measured over time with the substrate TMB (which is colorless but is converted into a blue product by oxidation through HRP). Furthermore, the addition of H2O2 catalyses the oxidation of the TMB since it acts as a electron donor, enhancing the blue product. The reaction can be stopped by adding an acidic solution (for example: HCl), which results in a yellow coloured-readout. | |
| − | + | See more information about the HRP activity in [https://parts.igem.org/Part:BBa_K1800002 BBa_K1800002] | |
| − | To prove the correct activity of the HRP in our Full Construct we performed mechanical lysis on | + | To prove the correct activity of the HRP in our Full Construct we used bacteria expressing it, performed mechanical lysis on them, added the HRP substrate TMB followed by the addition of H2O2 and stopped the reaction by adding HCl. Bacteria not expressing the Full Construct were used as a negative control. The result can be seen very nicely in the following video: |
[[File:Cuvetten_mod.mp4]] | [[File:Cuvetten_mod.mp4]] | ||
| − | To further verify the correct activity of the HRP, the absorbance at 650 nm of both cell lysates was | + | To further verify the correct activity of the HRP, the absorbance at 650 nm of both cell lysates was performed. As it can be seen in the following Figure, the cell lysate that is not expressing the Full Construct show a much lower absorbance at 650 nm, than the lysate containing the Full construct. This means, that the HRP contained in Full Construct is working properly (Figure 12). |
[[File:Cell_lysate_Full_Construct.png|center|300px|thumb|none|Figure 12: Absorbance measured at 650 nm of cell lysates with and without Full Construct after TMB addition. ]] | [[File:Cell_lysate_Full_Construct.png|center|300px|thumb|none|Figure 12: Absorbance measured at 650 nm of cell lysates with and without Full Construct after TMB addition. ]] | ||
| − | |||
===== c) dCas9 activity===== | ===== c) dCas9 activity===== | ||
| Line 95: | Line 154: | ||
===== d) Strep-tag column purification ===== | ===== d) Strep-tag column purification ===== | ||
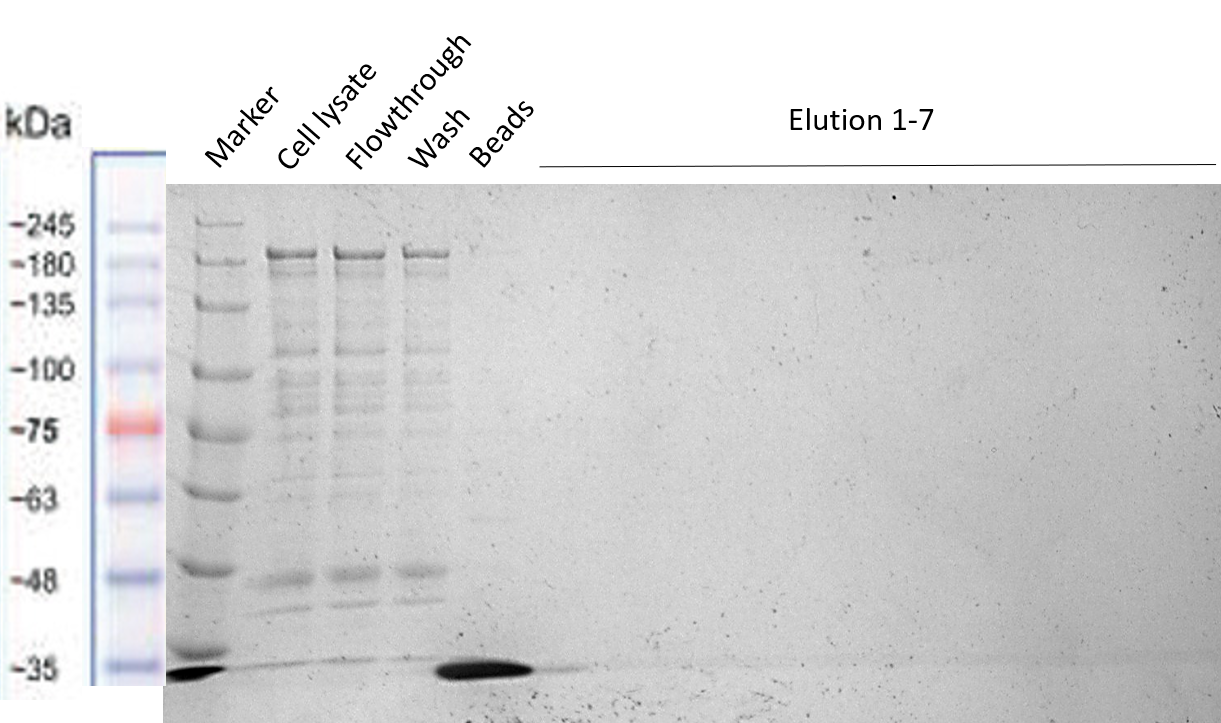
| − | The reason to include a Strep-tag at the end of our Full Construct was to facilitate its purification. However, as already explained in the Registry page of the Strep-tag itself [https://parts.igem.org/Part:BBa_K823038 BBa_K823038], this BioBrick seems to not be working properly for column purification. The Strep-tag | + | The reason to include a Strep-tag at the end of our Full Construct was to facilitate its purification. However, as already explained in the Registry page of the Strep-tag itself [https://parts.igem.org/Part:BBa_K823038 BBa_K823038], this BioBrick seems to not be working properly for column purification. The Strep-tag is probably meant to be used for Western Blots and not for column purification. That is why the purification via Strep-tag did not work (see Figure below). However, it was shown in the section before, that we were able to successfully purify it via the MBP (Figure 13/14). |
| − | [[File:Strep_purification_2.png|center|400px|thumb|none|Figure 14: Different Full Construct samples before, while and after Strep purification]] | + | [[File:Strep_purification_2.png|center|400px|thumb|none|Figure 13/14: Different Full Construct samples before, while and after Strep purification]] |
| + | |||
| + | == Sequence == | ||
| + | |||
| + | NOTE: Please be aware, that by combining all the basic parts used for this composite part, the registry automatically inserted a RFC23 scar. However, the design and our cloning strategy is based on RFC25. | ||
| + | |||
| + | |||
| + | <partinfo>BBa_K3037003 SequenceAndFeatures</partinfo> | ||
| + | |||
| + | == References == | ||
| + | |||
| + | [1] https://www.promega.com/~/media/files/resources/technical%20references/amino%20acid%20abbreviations%20and%20molecular%20weights.pdf | ||
Revision as of 22:20, 21 October 2019
Fusion protein dCas9 + HRP (MBP/dCas9/linker/HRP/Strep-tag)
| Fusion protein | |
|---|---|
| Function | Colour detection of specific DNA sequences |
| Use in | Escherichia coli |
| RFC standard | Freiburg RFC25 standard |
| Backbone | pSB1C3 |
| Experimental Backbone | pOCC97 |
| Submitted by | Team: TU_Dresden 2019 |
Contents
- 1 Overview
- 2 Biology
- 3 Characterization
- 3.1 Outline
- 3.2 Experiments in Detail
- 3.2.1 1) Expression of our Full Construct (FC) in pOCC97 (BBa_K3037000): and testing the growth of E.coli.
- 3.2.2 2) SDS-PAGEs showing the expression assay over time
- 3.2.3 3) Image analysis of the expression in the SDS-PAGEs with ImageJ
- 3.2.4 4) Characterization of the single parts of the full construct
- 4 Sequence
- 5 References
Overview
The TU Dresden 2019 team has designed this BioBrick in order to allow for the quick detection of specific DNA sequences of interest (more information).
The Full Construct as it is shown in Figure 1 below, contains five individual parts each of which have been optimized for it. The MBP and Strep-tag allow purification via amylose resin and Strep-columns, respectively. Additionally, the MBP enhances the expression of dCas9-fusion proteins, and the linker aids for the folding process. The dCas9 identifies the sequence of interest (with the help of an added guideRNA), and the HRP provides with an easily detectable amplified color readout. The single constructs were cloned into pSB1C3 and pSB1A3, and finally, the full construct was inserted into the BBa_K3037000 vector for expression and characterization in Escherichia coli (E. coli).
The weight of the fusion protein was calculated in accordance to the number of base pairs. The sequence of our Full Construct is 924 bp/3 = 308 amino acids long, each amino acid weights on average 110 Dalton [1], which results in the final weight of approximately 230 kDa.
Biology
For more information regarding the biology, design and function of each of the basic parts, please check the individual registry pages:
- MBP: BBa_K3037001
- dCas9: BBa_K3037002
- Linker: BBa_K3037004
- HRP: BBa_K3037007
- Strep-tag: BBa_K823038
The expected parameters of the new protein (determined with ExPASy ProtParam tool) were determined to be:
- Extinction coefficient: 205750 L/(mol*cm)
- Estimated half-life > 30 hours in mammalian cells, >20 hours in yeast and >10 hours in E. coli
Characterization
Outline
We performed the following characterization experiments:
1) Expression of our Full Construct (FC) in pOCC97 (BBa_K3037000): and testing the growth of E.coli.
2) SDS-PAGEs showing the expression over time
3) Image analysis of the expression in the SDS-PAGEs with ImageJ
4) Characterization of the single parts of the full construct
Experiments in Detail
1) Expression of our Full Construct (FC) in pOCC97 (BBa_K3037000): and testing the growth of E.coli.
The full construct, once all the single parts were fused together, was cloned into our expression plasmid K3037000 (p0CC97). The correct insertion of our full construct into the plasmid was proven via restriction digest followed by agarose gel electrophoresis. For that, we performed a triple digest with PmlI, X and P and got several positive clones. On the right, the simulation of the digest in SnapGene is shown (Figure 1).
Furthermore, it was proven that the E. coli could grow normally after the induction of the fusion protein. For that, the development of the bacteria cultures was monitored by measuring the OD at 600 nm during different time points before and after induction with 1 mM IPTG (Figure 2).
As shown in the curve, the growth of the bacteria is not affected by the expression of the protein. Important to note here is that the expression of the full construct was performed in two slightly different pOCC97 plasmids, that differ on their Ribosome Binding Site (RBS). Herein after they are going to be referred to as optimized and not optimized (read the registry page BBa_K3037000 to for more details regarding the difference between these two plasmids).
To go further, the expression of the full construct in pOCC97 at different temperatures was studied. For that, the optimized and not optimized pOOC97 were compared (Figure 3).
2) SDS-PAGEs showing the expression assay over time
After proving that the final construct was well inserted in our plasmid, the full construct was expressed overnight. The first expression was performed at 37°C for seven hours, induced with 1 mM IPTG. The result is shown below (Figure 4):
The same experiment was repeated several times at different temperatures and IPTG concentrations in both, the optimized and not optimized pOCC97 to compare the best expression conditions. The results are shown in the Figures below.
Expression of Full Construct in pOCC97 not optimized at 18ºC and different IPTG concentrations
Expression of Full Construct in pOCC97 not optimized at 37ºC and different IPTG concentrations
Expression of the Full Construct in pOCC97 optimized at different temperatures and IPTG concentrations
3) Image analysis of the expression in the SDS-PAGEs with ImageJ
The previously shown SDS-pages were then further analysed by using the software ImageJ to correct for loading differences and be able to draw conclusions about the best conditions to express the Full Construct in pOCC97.
Temperature and IPTG induction dependence of the optimized pOCC97
Temperature and IPTG induction dependence of the not optimized POCC97
Comparison between optimized and not optimized POCC97
Conclusion
Based on this analysis it can be concluded that the optimal conditions for the expression of BBa_3037003 in POCC97 are 18ºC and 0.5 mM IPTG. The expression seems to be more stable over time for the optimized plasmid than for the non-optimized.
4) Characterization of the single parts of the full construct
a) Purification via MBP
After proving that the Full Construct is expressed properly in our plasmid and improved its expression conditions, it was purified by using Amylose Resin to bind its MBP site. To test for the correct functioning of each single part of the fusion protein we performed different experiments. For that, two different protocols were used. On the one hand, an Amylose Resin column was used and on the other hand a batch binding solution was prepared. Better results were obtained with the latter one and therefore, in which the resin was pipetted into a falcon and incubated with the cell lysate for 1.5 hours on a rotator at 4°C (Figure 11).
b) Activity assay of HRP
Investigating the activity of HRP in the Full Construct was done in a dynamic assay. The absorbance at 650 nm was measured over time with the substrate TMB (which is colorless but is converted into a blue product by oxidation through HRP). Furthermore, the addition of H2O2 catalyses the oxidation of the TMB since it acts as a electron donor, enhancing the blue product. The reaction can be stopped by adding an acidic solution (for example: HCl), which results in a yellow coloured-readout. See more information about the HRP activity in BBa_K1800002 To prove the correct activity of the HRP in our Full Construct we used bacteria expressing it, performed mechanical lysis on them, added the HRP substrate TMB followed by the addition of H2O2 and stopped the reaction by adding HCl. Bacteria not expressing the Full Construct were used as a negative control. The result can be seen very nicely in the following video:
To further verify the correct activity of the HRP, the absorbance at 650 nm of both cell lysates was performed. As it can be seen in the following Figure, the cell lysate that is not expressing the Full Construct show a much lower absorbance at 650 nm, than the lysate containing the Full construct. This means, that the HRP contained in Full Construct is working properly (Figure 12).
c) dCas9 activity
d) Strep-tag column purification
The reason to include a Strep-tag at the end of our Full Construct was to facilitate its purification. However, as already explained in the Registry page of the Strep-tag itself BBa_K823038, this BioBrick seems to not be working properly for column purification. The Strep-tag is probably meant to be used for Western Blots and not for column purification. That is why the purification via Strep-tag did not work (see Figure below). However, it was shown in the section before, that we were able to successfully purify it via the MBP (Figure 13/14).
Sequence
NOTE: Please be aware, that by combining all the basic parts used for this composite part, the registry automatically inserted a RFC23 scar. However, the design and our cloning strategy is based on RFC25.
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 2302
Illegal NheI site found at 5500 - 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 381
Illegal BglII site found at 5742
Illegal BamHI site found at 4581
Illegal BamHI site found at 5314
Illegal XhoI site found at 5826 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 79