Difference between revisions of "Part:BBa K3254015"
| (7 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
<partinfo>BBa_K3254015 short</partinfo> | <partinfo>BBa_K3254015 short</partinfo> | ||
| − | This is a mutant Ptac promoter. The -10 region was mutated to GATACT from TATAAT, and has an "ideal" lac operator. This promoter has a lower leakage. | + | This is a mutant Ptac promoter based on the [[Part:BBa_K2572025|BBa_K2572025]]. The -10 region was mutated to GATACT from TATAAT, and has an "ideal" lac operator. This promoter has a lower leakage. |
| + | |||
| + | =Thermodynamic Characterization= | ||
| + | *This part was an derived version of the Ptac promoter ([[Part:BBa_K2572025|BBa_K2572025]]). | ||
| + | *We used this part to construct an IPTG inducible transcriptional device by combining with a lacI expression cassette([[Part:BBa_K3254022|BBa_K3254022]]) on a P15A plamisd. An insulated sfgfp reporter translational unit ([[Part:BBa_K3254024|BBa_K3254024]]) was applied for quantitative assay the dynamic response curve. The full structure of this operon was similar to [[Part:BBa_K3254025|BBa_K3254025]]. | ||
| + | *The series of IPTG inducible promoters with different dynamic ranges and non-induced activities include [[Part:BBa_K864400|Original Ptac]], [[Part:BBa_K2572025|Ptac]], [[Part:BBa_K3254015|Ptac-M1]], [[Part:BBa_K3254016|Ptac-M2]] and [[Part:BBa_K3254017|Ptac-M3]]. | ||
| + | |||
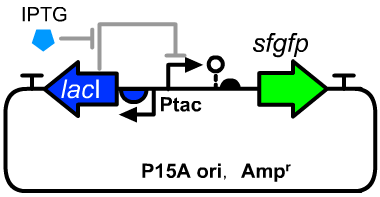
| + | ==Genetic Design== | ||
| + | [[File:T--GENAS_China--Ptac-sfGFP_plasmid.PNG|200px|thumb|left|The structure of the experimental plasmid]] | ||
| + | *The sequence detail was described on the page of [[Part:BBa_K3254025|BBa_K3254025]]. | ||
| + | *The host cell was E.coli DH5α. | ||
| + | |||
| + | ==Experimental Setup== | ||
| + | *All incubations were carried out using a Digital Thermostatic Shaker maintained at 37 °C and 1000 rpm, using Corning flat-bottom 96-well plates sealed with sealing film. For characterization the circuit response functions, a previously developed quantitative method that measures gene expression at steady state was used(Zhang, Chen et al. 2016). Briefly, bacteria harboring the parts/circuits of interest were first inoculated from single colonies into a flat-bottom 96-well plate for overnight growth, after which the cell cultures were diluted 196-fold with M9 medium. After 3 h of growth, the cultures were further diluted 700-fold with M9 medium containing gradient concentrations of IPTG, and incubated for another 6 h. Finally, 20-μL samples of each culture were transferred to a new plate containing 180 μL per well of PBS supplemented with 2 mg/mL kanamycin to terminate protein expression. The fluorescence distribution of each sample was assayed using a flow cytometer with appropriate voltage settings; each distribution contained >20,000 events. Each sample was experimentally assayed at least three times. The arithmetical mean of each sample was determined using FlowJo software. | ||
| + | *M9 medium (supplemented): 6.8 g/L Na<sub>2</sub>HPO<sub>4</sub>, 3 g/L KH<sub>2</sub>PO<sub>4</sub>, 0.5 g/L NaCl, 1 g/L NH<sub>4</sub>Cl, 0.34 g/L thiamine, 0.2% casamino acids, 0.4% glucose, 2mM MgSO<sub>4</sub>, and 100 μM CaCl<sub>2</sub>. | ||
| + | |||
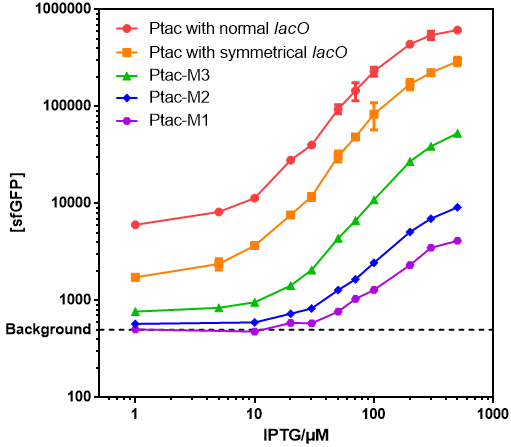
| + | ==Results== | ||
| + | *Those promoters had gentle response curves without saltus. | ||
| + | [[File:T--GENAS_China--IPTG-sfGFP.PNG]] | ||
| + | *Background: Cells without any FP genes. | ||
| + | *Ptac with normal lacO: [[Part:BBa_K864400|BBa_K864400]] | ||
| + | *Ptac with symmetrical lacO: [[Part:BBa_K2572025|BBa_K2572025]] | ||
| + | *Ptac-M1: [[Part:BBa_K3254015|BBa_K3254015]] | ||
| + | *Ptac-M2: [[Part:BBa_K3254016|BBa_K3254016]] | ||
| + | *Ptac-M3: [[Part:BBa_K3254017|BBa_K3254017]] | ||
| + | |||
| + | ==References== | ||
| + | *Zhang HM, et al. Measurements of Gene Expression at Steady State Improve the Predictability of Part Assembly. ACS Synthetic Biology 5, 269-273 (2016). | ||
| + | *Zong Y, et al. Insulated transcriptional elements enable precise design of genetic circuits. Nature communications 8, 52 (2017). | ||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
Latest revision as of 11:44, 21 October 2019
Mutant Ptac promoter No.1
This is a mutant Ptac promoter based on the BBa_K2572025. The -10 region was mutated to GATACT from TATAAT, and has an "ideal" lac operator. This promoter has a lower leakage.
Thermodynamic Characterization
- This part was an derived version of the Ptac promoter (BBa_K2572025).
- We used this part to construct an IPTG inducible transcriptional device by combining with a lacI expression cassette(BBa_K3254022) on a P15A plamisd. An insulated sfgfp reporter translational unit (BBa_K3254024) was applied for quantitative assay the dynamic response curve. The full structure of this operon was similar to BBa_K3254025.
- The series of IPTG inducible promoters with different dynamic ranges and non-induced activities include Original Ptac, Ptac, Ptac-M1, Ptac-M2 and Ptac-M3.
Genetic Design
- The sequence detail was described on the page of BBa_K3254025.
- The host cell was E.coli DH5α.
Experimental Setup
- All incubations were carried out using a Digital Thermostatic Shaker maintained at 37 °C and 1000 rpm, using Corning flat-bottom 96-well plates sealed with sealing film. For characterization the circuit response functions, a previously developed quantitative method that measures gene expression at steady state was used(Zhang, Chen et al. 2016). Briefly, bacteria harboring the parts/circuits of interest were first inoculated from single colonies into a flat-bottom 96-well plate for overnight growth, after which the cell cultures were diluted 196-fold with M9 medium. After 3 h of growth, the cultures were further diluted 700-fold with M9 medium containing gradient concentrations of IPTG, and incubated for another 6 h. Finally, 20-μL samples of each culture were transferred to a new plate containing 180 μL per well of PBS supplemented with 2 mg/mL kanamycin to terminate protein expression. The fluorescence distribution of each sample was assayed using a flow cytometer with appropriate voltage settings; each distribution contained >20,000 events. Each sample was experimentally assayed at least three times. The arithmetical mean of each sample was determined using FlowJo software.
- M9 medium (supplemented): 6.8 g/L Na2HPO4, 3 g/L KH2PO4, 0.5 g/L NaCl, 1 g/L NH4Cl, 0.34 g/L thiamine, 0.2% casamino acids, 0.4% glucose, 2mM MgSO4, and 100 μM CaCl2.
Results
- Those promoters had gentle response curves without saltus.
- Background: Cells without any FP genes.
- Ptac with normal lacO: BBa_K864400
- Ptac with symmetrical lacO: BBa_K2572025
- Ptac-M1: BBa_K3254015
- Ptac-M2: BBa_K3254016
- Ptac-M3: BBa_K3254017
References
- Zhang HM, et al. Measurements of Gene Expression at Steady State Improve the Predictability of Part Assembly. ACS Synthetic Biology 5, 269-273 (2016).
- Zong Y, et al. Insulated transcriptional elements enable precise design of genetic circuits. Nature communications 8, 52 (2017).
Sequence and Features
Assembly Compatibility:
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]