Difference between revisions of "Part:BBa K2924004"
| Line 8: | Line 8: | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
<html><p align="justify"> </html> | <html><p align="justify"> </html> | ||
| − | This part contains the <i>BRYFOR_06758</i> gene coding for the acyl-[acyl-carrier-protein] thioesterase of <i>Marvinbryantia formatexigens</i>, which can be found under the UniProt ID: C6LDQ9_9FIRM and shows a thiolester hydrolase activity<sup> | + | This part contains the <i>BRYFOR_06758</i> gene coding for the acyl-[acyl-carrier-protein] thioesterase of <i>Marvinbryantia formatexigens</i>, which can be found under the UniProt ID: C6LDQ9_9FIRM and shows a thiolester hydrolase activity<sup>1</sup>. |
====Background==== | ====Background==== | ||
| Line 18: | Line 18: | ||
<html><p align="justify"> </html> | <html><p align="justify"> </html> | ||
| − | Butyric acid (Fig. 1) is a straight-chained saturated 4:0 fatty acid. It is an oily and colorless liquid and has a unpleasant, rancid odor <sup> | + | Butyric acid (Fig. 1) is a straight-chained saturated 4:0 fatty acid. It is an oily and colorless liquid and has a unpleasant, rancid odor <sup>2</sup>. It has a molecular mass of 88.11 g/mol. Butyric acid is found in animal fat and plant oils <sup>2</sup>, bovine milk, butter, cheese, and human breast milk <sup>3</sup>. |
<html><p align="justify"> </html> | <html><p align="justify"> </html> | ||
| − | Hexanoic acid (Fig. 2) is a straight-chained saturated 6:0 fatty acid, which is also called caproic acid. It appears as a white crystalline solid and has a unpleasant odor <sup> | + | Hexanoic acid (Fig. 2) is a straight-chained saturated 6:0 fatty acid, which is also called caproic acid. It appears as a white crystalline solid and has a unpleasant odor <sup>4</sup>. It has a molecular mass of 116,16 g/mol. It is found naturally in animal fats and oils and various plants <sup>4</sup>. |
<html><p align="justify"> </html> | <html><p align="justify"> </html> | ||
| − | Octanoic acid (Fig. 3) is a saturated fatty acid with 8 carbon atoms, which is also called caprylic acid. It is a colorless<sup> | + | Octanoic acid (Fig. 3) is a saturated fatty acid with 8 carbon atoms, which is also called caprylic acid. It is a colorless<sup>5</sup>/light yellow liquid and has a mild to fruity-acid odor <sup>6</sup>. It has a molecular mass of 144,21 g/mol. |
| Line 31: | Line 31: | ||
Fatty acids are synthesized and elongated by fatty acid synthases, which is a complex containing multiple enzymes. The determination of its length is controlled by thioesterases, a subgroup of hydrolases. They hydrolyze acetyl-CoA esters or acyl carrier protein esters to the corresponding free fatty acid and the Coenzyme A or the acyl carrier protein. | Fatty acids are synthesized and elongated by fatty acid synthases, which is a complex containing multiple enzymes. The determination of its length is controlled by thioesterases, a subgroup of hydrolases. They hydrolyze acetyl-CoA esters or acyl carrier protein esters to the corresponding free fatty acid and the Coenzyme A or the acyl carrier protein. | ||
<html><p align="justify"> </html> | <html><p align="justify"> </html> | ||
| − | The acyl-[acyl-carrier-protein] thioesterase of <i>Marvinbryantia formatexigens</i> has been tested to show catalytic activities in producing a high amount of 4:0, 6:0 8:0 <sup> | + | The acyl-[acyl-carrier-protein] thioesterase of <i>Marvinbryantia formatexigens</i> has been tested to show catalytic activities in producing a high amount of 4:0, 6:0 8:0 <sup>7</sup>. <i>M. formatexigens</i> use cellulose and hemicellulose in vegetables <sup>8</sup>, which is digested in the human colon by a microbial community including <i>M. formatexigens</i>, as source for production of short-chain fatty acids <sup>8</sup>. |
====Use of butyric acid, hexanoic acid and octanoic acid==== | ====Use of butyric acid, hexanoic acid and octanoic acid==== | ||
<html><p align="justify"> </html> | <html><p align="justify"> </html> | ||
| − | By engineering metabolic pathways to produce designer fatty acids with the correct amount of carbons in the chain, such fatty acids could be used directly for chemicals or fuels with less processing <sup> | + | By engineering metabolic pathways to produce designer fatty acids with the correct amount of carbons in the chain, such fatty acids could be used directly for chemicals or fuels with less processing <sup>9, 10</sup> and less usage of fossil resources. Therefore, it is a step towards a environmental friendly alternative <sup>11</sup>. |
<html><p align="justify"> </html> | <html><p align="justify"> </html> | ||
| − | Butyric acid has a broad range of usage in chemical and fuel industries <sup> | + | Butyric acid has a broad range of usage in chemical and fuel industries <sup>10, 12</sup>. In manufacture, it is used as artificial flavoring ingredient for candies, certain liquors or syrups <sup>2</sup>. |
<html><p align="justify"> </html> | <html><p align="justify"> </html> | ||
| − | Hexanoic acid has several applications. It is related to tobacco products, to cleaning and washing material, to colorants and dyes, and is used as a general flavoring agent for food<sup> | + | Hexanoic acid has several applications. It is related to tobacco products, to cleaning and washing material, to colorants and dyes, and is used as a general flavoring agent for food<sup>4</sup>. |
<html><p align="justify"> </html> | <html><p align="justify"> </html> | ||
| − | Octanoic acid is added directly to human food affirmed as generally recognized as safe (GRAS) <sup> | + | Octanoic acid is added directly to human food affirmed as generally recognized as safe (GRAS) <sup>13, 14</sup>. The substance is used as a flavoring agent and adjuvant and occurs normally in various foods, like baked goods, cheeses, fats and oils, frozen dairy desserts, gelatins and puddings, soft candy and snack foods. It is found in milk of various mammals and is a minor component of coconut oil and palm kernel oil <sup>15</sup>. Therefore, it has a low toxicity ( oral LD50 for rat: 10.08 g/kg <sup>13</sup>). |
<br><br><br><br><br><br><br><br><br><br> | <br><br><br><br><br><br><br><br><br><br> | ||
| Line 66: | Line 66: | ||
===References=== | ===References=== | ||
| − | [ | + | [1]: https://www.uniprot.org/uniprot/C6LDQ9 |
| − | [ | + | [2]: National Center for Biotechnology Information. PubChem Database. Butyric acid, CID=264, https://pubchem.ncbi.nlm.nih.gov/compound/Butyric-acid (accessed on Sept. 20, 2019) |
| − | [ | + | [3]: McNabney, Sean M., and Tara M. Henagan. "Short chain fatty acids in the colon and peripheral tissues: a focus on butyrate, colon cancer, obesity and insulin resistance." <i>Nutrients</i> 9.12 (2017): 1348. |
| − | [ | + | [4]: National Center for Biotechnology Information. PubChem Database. Hexanoic acid, CID=8892, https://pubchem.ncbi.nlm.nih.gov/compound/Hexanoic-acid (accessed on Sept. 20, 2019) |
| − | [ | + | [5]: Lewis, R.J. Sr.; Hawley's Condensed Chemical Dictionary 14th Edition. John Wiley & Sons, Inc. New York, NY 2001., p. 286 |
| − | [ | + | [6]: Fenaroli's Handbook of Flavor Ingredients. Volume 2. Edited, translated, and revised by T.E. Furia and N. Bellanca. 2nd ed. Cleveland: The Chemical Rubber Co., 1975., p. 442 |
| − | [ | + | [7]:Jing, F., Cantu, D. C., Tvaruzkova, J., Chipman, J. P., Nikolau, B. J., Yandeau-Nelson, M. D., & Reilly, P. J. (2011). Phylogenetic and experimental characterization of an acyl-ACP thioesterase family reveals significant diversity in enzymatic specificity and activity. BMC biochemistry, 12(1), 44. |
| − | [ | + | [8]: Wolin, M. J., Miller, T. L., Collins, M. D., & Lawson, P. A. (2003). Formate-Dependent Growth and Homoacetogenic Fermentation by a Bacterium from Human Feces: Description of Bryantella formatexigens gen. nov., sp. nov. Appl. Environ. Microbiol., 69(10), 6321-6326 |
| − | [ | + | [9]: Ziesack, M., Rollins, N., Shah, A., Dusel, B., Webster, G., Silver, P. A., & Way, J. C. (2018). Chimeric fatty acyl-acyl carrier protein thioesterases provide mechanistic insight into enzyme specificity and expression. Appl. Environ. Microbiol., 84(10), e02868-17. |
| − | [ | + | [10]: Jawed, K., Mattam, A. J., Fatma, Z., Wajid, S., Abdin, M. Z., & Yazdani, S. S. (2016). Engineered production of short chain fatty acid in Escherichia coli using fatty acid synthesis pathway. PloS one, 11(7), e0160035. |
| − | [ | + | [11]: Baroi, G. N., Baumann, I., Westermann, P., & Gavala, H. N. (2015). Butyric acid fermentation from pretreated and hydrolysed wheat straw by an adapted C lostridium tyrobutyricum strain. Microbial biotechnology, 8(5), 874-882. |
| − | [ | + | [12]: Liu, X., Yu, H., Jiang, X., Ai, G., Yu, B., & Zhu, K. (2015). Biosynthesis of butenoic acid through fatty acid biosynthesis pathway in Escherichia coli. Applied microbiology and biotechnology, 99(4), 1795-1804. |
| − | [ | + | [13]: National Center for Biotechnology Information. PubChem Database. Octanoic acid, CID=379, https://pubchem.ncbi.nlm.nih.gov/compound/Octanoic-acid (accessed on Sept. 19, 2019) |
| − | [ | + | [14]: U.S. Food and Drug: “Food Additive Status List” https://www.fda.gov/food/food-additives-petitions/food-additive-status-list (accessed on Oct. 2, 2019) |
| − | [ | + | [15]: Federal Government: “Caprylic acid” https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=1&SID=02ff2ac4eea1218953e3565902ed9655&ty=HTML&h=L&mc=true&r=SECTION&n=se21.3.184_11025 (accessed on Oct. 2, 2019) |
Latest revision as of 19:12, 20 October 2019
Thioesterase from Marvinbryantia formatexigens
Acyl-[acyl-carrier-protein] thioesterase BRYFOR_06758 from Marvinbryantia formatexigens
Usage and Biology
This part contains the BRYFOR_06758 gene coding for the acyl-[acyl-carrier-protein] thioesterase of Marvinbryantia formatexigens, which can be found under the UniProt ID: C6LDQ9_9FIRM and shows a thiolester hydrolase activity1.
Background
Fatty acids are long aliphatic chained carboxylic acids, which can be saturated or unsaturated. They have mostly an even number of carbon atoms from 4 to 28.
Butyric acid (Fig. 1) is a straight-chained saturated 4:0 fatty acid. It is an oily and colorless liquid and has a unpleasant, rancid odor 2. It has a molecular mass of 88.11 g/mol. Butyric acid is found in animal fat and plant oils 2, bovine milk, butter, cheese, and human breast milk 3.
Hexanoic acid (Fig. 2) is a straight-chained saturated 6:0 fatty acid, which is also called caproic acid. It appears as a white crystalline solid and has a unpleasant odor 4. It has a molecular mass of 116,16 g/mol. It is found naturally in animal fats and oils and various plants 4.
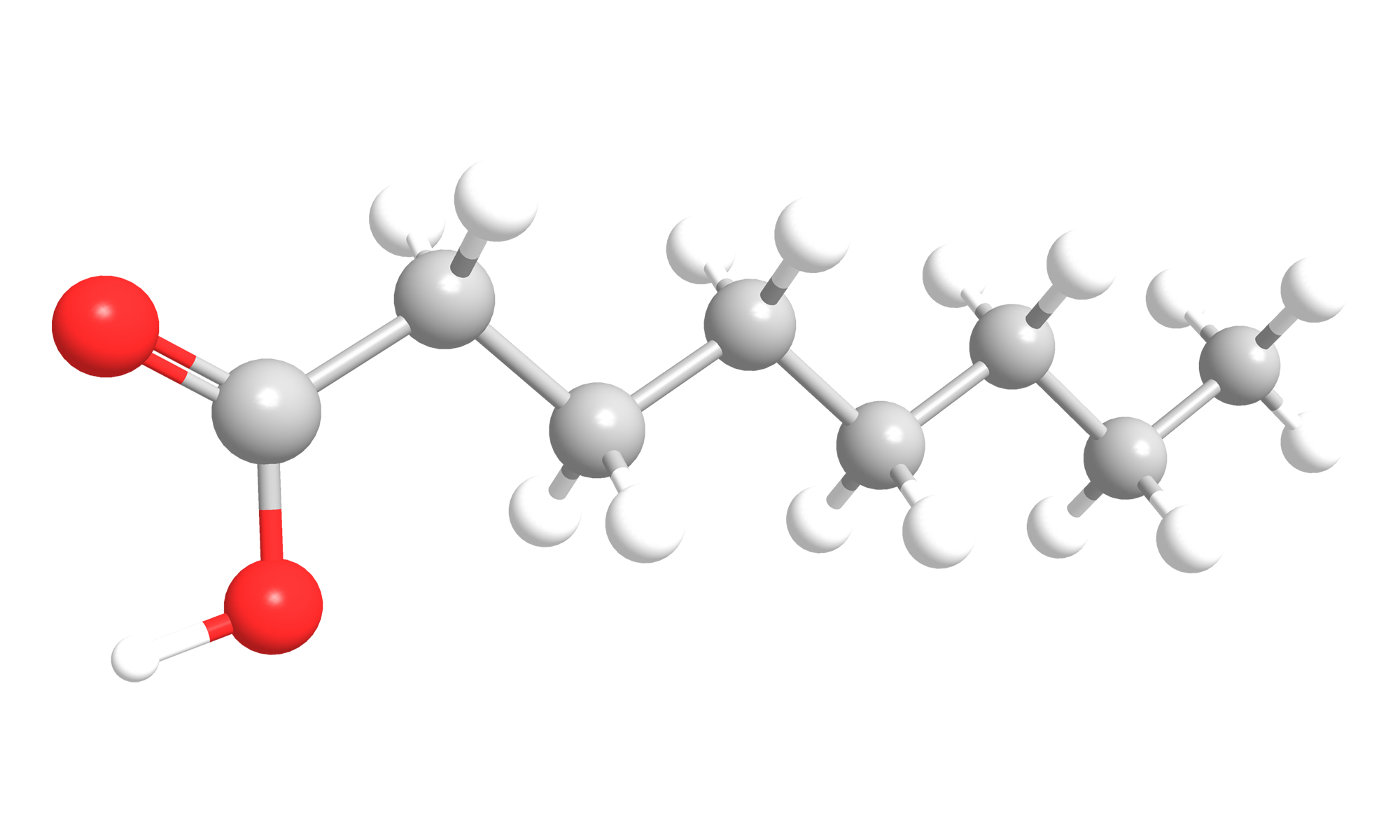
Octanoic acid (Fig. 3) is a saturated fatty acid with 8 carbon atoms, which is also called caprylic acid. It is a colorless5/light yellow liquid and has a mild to fruity-acid odor 6. It has a molecular mass of 144,21 g/mol.
Biosynthesis
Fatty acids are synthesized and elongated by fatty acid synthases, which is a complex containing multiple enzymes. The determination of its length is controlled by thioesterases, a subgroup of hydrolases. They hydrolyze acetyl-CoA esters or acyl carrier protein esters to the corresponding free fatty acid and the Coenzyme A or the acyl carrier protein.
The acyl-[acyl-carrier-protein] thioesterase of Marvinbryantia formatexigens has been tested to show catalytic activities in producing a high amount of 4:0, 6:0 8:0 7. M. formatexigens use cellulose and hemicellulose in vegetables 8, which is digested in the human colon by a microbial community including M. formatexigens, as source for production of short-chain fatty acids 8.
Use of butyric acid, hexanoic acid and octanoic acid
By engineering metabolic pathways to produce designer fatty acids with the correct amount of carbons in the chain, such fatty acids could be used directly for chemicals or fuels with less processing 9, 10 and less usage of fossil resources. Therefore, it is a step towards a environmental friendly alternative 11.
Butyric acid has a broad range of usage in chemical and fuel industries 10, 12. In manufacture, it is used as artificial flavoring ingredient for candies, certain liquors or syrups 2.
Hexanoic acid has several applications. It is related to tobacco products, to cleaning and washing material, to colorants and dyes, and is used as a general flavoring agent for food4.
Octanoic acid is added directly to human food affirmed as generally recognized as safe (GRAS) 13, 14. The substance is used as a flavoring agent and adjuvant and occurs normally in various foods, like baked goods, cheeses, fats and oils, frozen dairy desserts, gelatins and puddings, soft candy and snack foods. It is found in milk of various mammals and is a minor component of coconut oil and palm kernel oil 15. Therefore, it has a low toxicity ( oral LD50 for rat: 10.08 g/kg 13).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Characterization
Note: This data belongs to this basic part, but was collected with the composite part BBa_K2924013 , containing the promoter BBa_J23119, the RBS* BBa_K2924009 and the double terminator BBa_B0015.
Unfortunately, we could not test the Escherichia coli transformants or the Synechocystis conjugants with (GC-MS) due to the unavailability of a method for short-chain fatty acid detection. This experiments can be conducted as soon as a suitable method is created.
References
[1]: https://www.uniprot.org/uniprot/C6LDQ9
[2]: National Center for Biotechnology Information. PubChem Database. Butyric acid, CID=264, https://pubchem.ncbi.nlm.nih.gov/compound/Butyric-acid (accessed on Sept. 20, 2019)
[3]: McNabney, Sean M., and Tara M. Henagan. "Short chain fatty acids in the colon and peripheral tissues: a focus on butyrate, colon cancer, obesity and insulin resistance." Nutrients 9.12 (2017): 1348.
[4]: National Center for Biotechnology Information. PubChem Database. Hexanoic acid, CID=8892, https://pubchem.ncbi.nlm.nih.gov/compound/Hexanoic-acid (accessed on Sept. 20, 2019)
[5]: Lewis, R.J. Sr.; Hawley's Condensed Chemical Dictionary 14th Edition. John Wiley & Sons, Inc. New York, NY 2001., p. 286
[6]: Fenaroli's Handbook of Flavor Ingredients. Volume 2. Edited, translated, and revised by T.E. Furia and N. Bellanca. 2nd ed. Cleveland: The Chemical Rubber Co., 1975., p. 442
[7]:Jing, F., Cantu, D. C., Tvaruzkova, J., Chipman, J. P., Nikolau, B. J., Yandeau-Nelson, M. D., & Reilly, P. J. (2011). Phylogenetic and experimental characterization of an acyl-ACP thioesterase family reveals significant diversity in enzymatic specificity and activity. BMC biochemistry, 12(1), 44.
[8]: Wolin, M. J., Miller, T. L., Collins, M. D., & Lawson, P. A. (2003). Formate-Dependent Growth and Homoacetogenic Fermentation by a Bacterium from Human Feces: Description of Bryantella formatexigens gen. nov., sp. nov. Appl. Environ. Microbiol., 69(10), 6321-6326
[9]: Ziesack, M., Rollins, N., Shah, A., Dusel, B., Webster, G., Silver, P. A., & Way, J. C. (2018). Chimeric fatty acyl-acyl carrier protein thioesterases provide mechanistic insight into enzyme specificity and expression. Appl. Environ. Microbiol., 84(10), e02868-17.
[10]: Jawed, K., Mattam, A. J., Fatma, Z., Wajid, S., Abdin, M. Z., & Yazdani, S. S. (2016). Engineered production of short chain fatty acid in Escherichia coli using fatty acid synthesis pathway. PloS one, 11(7), e0160035.
[11]: Baroi, G. N., Baumann, I., Westermann, P., & Gavala, H. N. (2015). Butyric acid fermentation from pretreated and hydrolysed wheat straw by an adapted C lostridium tyrobutyricum strain. Microbial biotechnology, 8(5), 874-882.
[12]: Liu, X., Yu, H., Jiang, X., Ai, G., Yu, B., & Zhu, K. (2015). Biosynthesis of butenoic acid through fatty acid biosynthesis pathway in Escherichia coli. Applied microbiology and biotechnology, 99(4), 1795-1804.
[13]: National Center for Biotechnology Information. PubChem Database. Octanoic acid, CID=379, https://pubchem.ncbi.nlm.nih.gov/compound/Octanoic-acid (accessed on Sept. 19, 2019)
[14]: U.S. Food and Drug: “Food Additive Status List” https://www.fda.gov/food/food-additives-petitions/food-additive-status-list (accessed on Oct. 2, 2019)
[15]: Federal Government: “Caprylic acid” https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=1&SID=02ff2ac4eea1218953e3565902ed9655&ty=HTML&h=L&mc=true&r=SECTION&n=se21.3.184_11025 (accessed on Oct. 2, 2019)