Difference between revisions of "Part:BBa K3182104"
| Line 1: | Line 1: | ||
| + | __TOC__ | ||
| − | + | <span class='h3bb'><h1>Sequence and Features</h1></span> | |
| − | + | <partinfo>BBa_K3182108 SequenceAndFeatures</partinfo> | |
| − | <h1> | + | |
| − | + | ||
| − | + | ||
| − | + | ||
<br> | <br> | ||
| − | < | + | <h1>Introduction</h1> |
| − | + | ||
| − | + | <partinfo>BBa_K3182108 short</partinfo>[[File:T--Linkoping_Sweden--fusionproteinillustration.jpg|420px|thumb|right|<b>Figure 1.</b> Mechanism of action. The CBDcipA-fusion is attached to cellulose. By adding thrombin from any source the fusion protein will be cleaved and the C-terminal fusion protein will be released into the solution. By changing the fusion protein to an antimicrobial peptide/enzyme, and using the cellulose as a bandage, the peptide/enzyme can be released into a wound by native human thrombin.]] | |
| − | < | + | <h3>Assembly compabilities</h3> |
| + | An internal BamHI recognition sequence (RS) has been added to enable changeable fusion proteins. BamHI was chosen because its RS codes for glycine and serine, fitting it to the end of the thrombin site. It is also cost-effective enzyme and is unaffected by methylated DNA. | ||
| − | + | This part can be used to track purification, measure CBD binding ability and report cleavage at the thrombin site. | |
| − | + | ||
| − | + | <br><br><br><br><br> | |
| + | <h2>CBDcipA crystal structure</h2> | ||
| − | < | + | [[File:T--Linkoping_Sweden--rotatingcbdanimationloop.gif|420px|thumb|left|<b>Figure 1.</b> Crystal structure of CBDcipA with a resolution of 1.75 Å which were solved by [http://www.ncbi.nlm.nih.gov/pmc/PMC452321 Tormo et al. 1989]. PDB code 1NBC. In red from the left, W118, R112, D56, H57 and Y67, thought to be the surface which interacts strongly with cellulose.]] |
| − | + | ||
| − | + | <h3>Important molecular faces</h3> | |
| + | CBDcipA is composed of a nine-stranded beta sandwich with a jelly roll topology and binds a calcium ion. It further contains conserved residues exposed on the surface which map into two clear surfaces on each side of the molecule. One of faces mainly contains planar strips of aromatic and polar residues which may be the cellulose binding part. Further aspect are unknown and unique with this CBD such as the other conserved residues which are contained in a groove. | ||
| + | |||
| + | <h3>The choice of cellulose binding domain</h3> | ||
| + | iGEM Linköping 2019 choose CBDcipA due to many other iGEM teams exploring the possibilities of this domain. Our basic design was influenced by iGEM14 Imperial, iGEM15 Edinburgh and iGEM18 Ecuador. Purification and where to place the fusion protein (N- or C-terminal) was determined by studying the former projects. CBDcipA also originates from a thermophilic bacteria which further increases the domains applications. | ||
| + | |||
| + | |||
| + | <br><br><br><br><br><br><br><br> | ||
| + | <h2>Expression system</h2> | ||
| − | + | The part has a very strong expression with a T7-RNA-polymerase promotor (<partinfo>BBa_I719005</partinfo>) as well as a 5'-UTR (<partinfo>BBa_K1758100</partinfo>) region which has been shown to further increase expression in E. coli (<partinfo>BBa_K1758106</partinfo>), ([http://www.ncbi.nlm.nih.gov/pubmed/2676996 Olins et al. 1989]), ([http://www.ncbi.nlm.nih.gov/pubmed/23927491 Takahashi et al. 2013]). Both this part and the part were sfGFP was changed for AsPink (<partinfo>BBa_K3182000</partinfo>) showed great expression. | |
| − | + | ||
| − | + | [[File:T--Linkoping_Sweden--expression.png|900px|thumb|center|<b>Figure B.</b> Benchling screenshot of the expression system. The T7-RNA-polymerase promotor is followed by a T7 g10 leader sequence which enhances the binding to the 16S ribosomal RNA. After the leader sequence a poly A spacer is found, which has been shown to increase translation in vitro. Before the start codon a strong RBS, g10-L, followed by an AT-rich spacer can be seen, which will slightly increase translation of the following gene.]] | |
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
| Line 40: | Line 43: | ||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
===Functional Parameters=== | ===Functional Parameters=== | ||
| − | |||
<!-- --> | <!-- --> | ||
Revision as of 16:08, 6 September 2019
Contents
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 580
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 598
Introduction
pT7-CBDcipA-sfGFP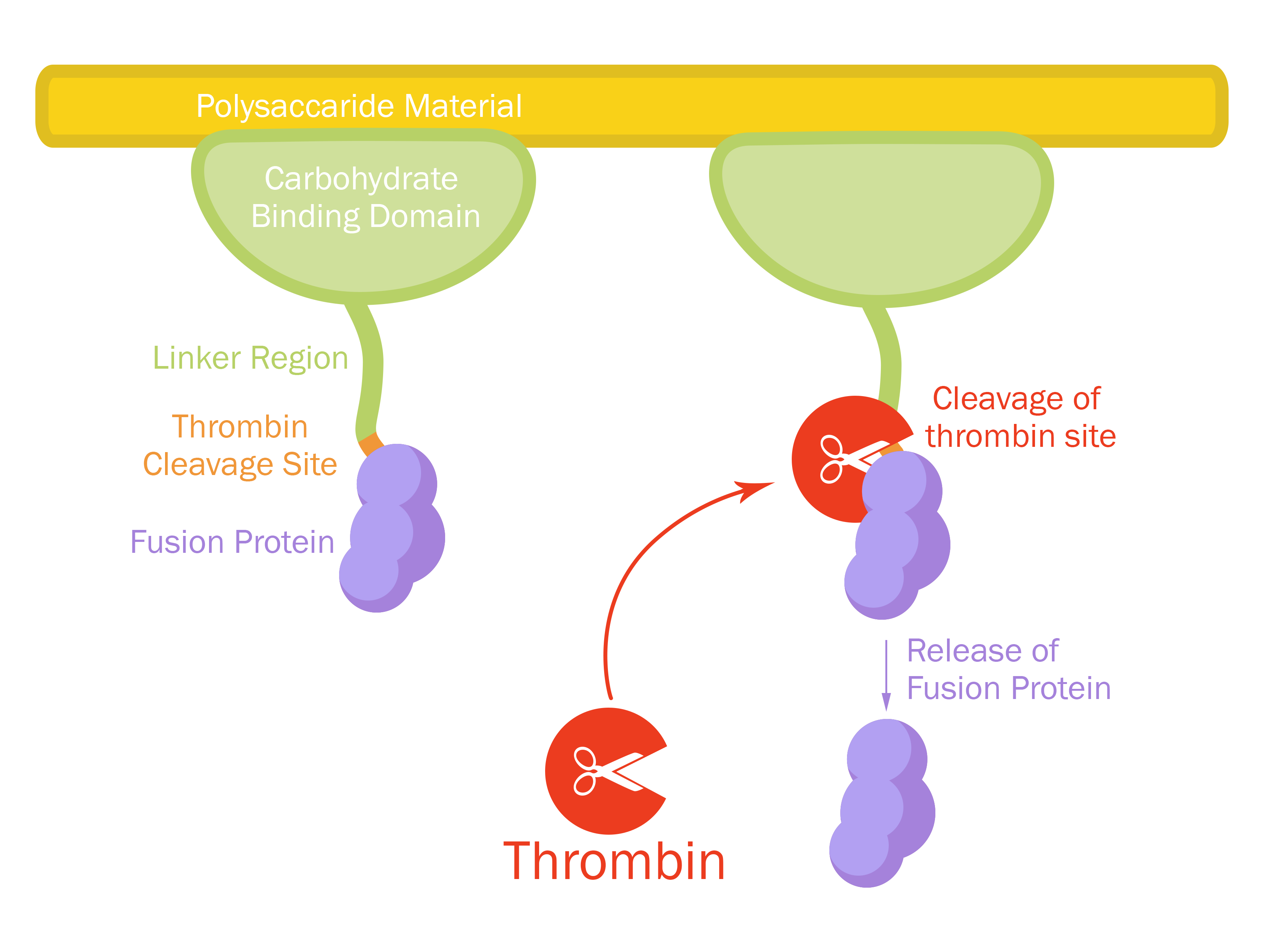
Assembly compabilities
An internal BamHI recognition sequence (RS) has been added to enable changeable fusion proteins. BamHI was chosen because its RS codes for glycine and serine, fitting it to the end of the thrombin site. It is also cost-effective enzyme and is unaffected by methylated DNA.
This part can be used to track purification, measure CBD binding ability and report cleavage at the thrombin site.
CBDcipA crystal structure
Important molecular faces
CBDcipA is composed of a nine-stranded beta sandwich with a jelly roll topology and binds a calcium ion. It further contains conserved residues exposed on the surface which map into two clear surfaces on each side of the molecule. One of faces mainly contains planar strips of aromatic and polar residues which may be the cellulose binding part. Further aspect are unknown and unique with this CBD such as the other conserved residues which are contained in a groove.
The choice of cellulose binding domain
iGEM Linköping 2019 choose CBDcipA due to many other iGEM teams exploring the possibilities of this domain. Our basic design was influenced by iGEM14 Imperial, iGEM15 Edinburgh and iGEM18 Ecuador. Purification and where to place the fusion protein (N- or C-terminal) was determined by studying the former projects. CBDcipA also originates from a thermophilic bacteria which further increases the domains applications.
Expression system
The part has a very strong expression with a T7-RNA-polymerase promotor (BBa_I719005) as well as a 5'-UTR (BBa_K1758100) region which has been shown to further increase expression in E. coli (BBa_K1758106), ([http://www.ncbi.nlm.nih.gov/pubmed/2676996 Olins et al. 1989]), ([http://www.ncbi.nlm.nih.gov/pubmed/23927491 Takahashi et al. 2013]). Both this part and the part were sfGFP was changed for AsPink (BBa_K3182000) showed great expression.

Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 580
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]

