Difference between revisions of "Part:BBa K2549039"
(→Biology) |
(→It works as we designed) |
||
| (8 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
<partinfo>BBa_K2549039 short</partinfo> | <partinfo>BBa_K2549039 short</partinfo> | ||
| − | This part is one of the downstream elements of our amplifier. It | + | This part is one of the downstream elements of our amplifier. It was constructed by fusing VP64 ([[Part:BBa_K2549057]]), dNLS ([[Part:BBa_K2549056]]) and ZF21.16 ([[Part:BBa_K2549046]]), from N terminal to C terminal. VP64 is a tetrameric VP16 transcription activator which shows ultrahigh transcription activation function. dNLS, which is short for destroyable nuclear localization sequence, is able to guide the fusion protein to be located to the nucleus without TEV protease. It is cleaved and unable to guide the fusion protein when TEV protease exist. When coexpressed with NLS-TEVp ([[Part:BBa_K2549041]]), it will be cleaved and the transcription activation function cannot be conducted. It is available to wire more complex binary logic gates including XOR gate and XNOR gate due to its wonderful feature. |
| + | |||
| + | <!-- --> | ||
| + | <span class='h3bb'>Sequence and Features</span> | ||
| + | <partinfo>BBa_K2549039 SequenceAndFeatures</partinfo> | ||
| + | |||
<!-- Add more about the biology of this part here --> | <!-- Add more about the biology of this part here --> | ||
===Biology=== | ===Biology=== | ||
| − | + | Please visit http://2018.igem.org/Team:Fudan/Results and http://2018.igem.org/Team:Fudan/Measurement to check how we use this. | |
| − | + | ||
| − | + | =====TEV protease-based transcription regulation or proteolysis-only activity===== | |
| − | + | TEV protease is widely used in synthetic biology for its high cleavage specificity (targetting amino acids sequence ENLYFQG/S between QG or QS)<ref>Release of proteins and peptides from fusion proteins using a recombinant plant virus proteinase. Parks TD, Leuther KK, Howard ED, Johnston SA, Dougherty WG. Anal Biochem, 1994 Feb;216(2):413-7 PMID: 8179197; DOI: 10.1006/abio.1994.1060</ref> and cleaves and high efficiency (optimized by Kapust RB et al to remove autolysis)<ref>Tobacco etch virus protease: mechanism of autolysis and rational design of stable mutants with wild-type catalytic proficiency. Kapust RB, Tözsér J, Fox JD, ..., Copeland TD, Waugh DS. Protein Eng, 2001 Dec;14(12):993-1000 PMID: 11809930</ref>. Wehr MC et al have demonstrated the TEV activity-dependent activation of several reporters<ref>Monitoring regulated protein-protein interactions using split TEV. Wehr MC, Laage R, Bolz U, ..., Nave KA, Rossner MJ. Nat Methods, 2006 Dec;3(12):985-93 PMID: 17072307; DOI: 10.1038/nmeth967</ref>. In our system, we set the '''TEV protease specific cleavage site into the spacer region''' of the nuclear localization sequence<ref>Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Robbins J, Dilworth SM, Laskey RA, Dingwall C. Cell, 1991 Feb;64(3):615-23 PMID: 1991323</ref>, which is the critical design of our TEV protease-based complex logic gates. | |
| + | [[File:TEVp.jpg|none|400px|thumb|Wehr MC et al stated:''TEV protease activity can be monitored with either inactivated transcription factors (‘transcription-coupled’ reporters) or inactivated reporter proteins (‘proteolysis-only’ reporters). We designed a ‘transcription-coupled’ TEV reporter at the membrane (TM-GV) by fusing a transcription factor composed of a yeast Gal4 DNA-binding domain and the herpes simplex VP16 transactivation domain (GV) to an hemagglutinin (HA)-tagged transmembrane region from the human PDGF-a receptor via a TEV-protease cleavage site ENLYFQ'G . After TEV protease cleavage, GV can translocate into the nucleus and induce reporter gene expression via five clustered Gal4-responsive cis elements. We constructed membrane localized ‘proteolysis-only’ reporters containing a luciferase moiety. We flanked GV N- and C-terminally by tevS sites and ERT2 domains (GV-2ER) thereby trapping the GV transcription factor reporter unit in the cytoplasm.'']] | ||
| − | + | For more details about TEV protease, please refer to our TEVp ([[Part:BBa_K2549013]]). | |
| − | + | ||
| − | + | ||
| Line 22: | Line 25: | ||
===Functional Parameters=== | ===Functional Parameters=== | ||
<partinfo>BBa_K2549039 parameters</partinfo> | <partinfo>BBa_K2549039 parameters</partinfo> | ||
| − | + | --> | |
| + | ===References=== | ||
Latest revision as of 21:35, 17 October 2018
VP64-dNLS-ZF21.16
This part is one of the downstream elements of our amplifier. It was constructed by fusing VP64 (Part:BBa_K2549057), dNLS (Part:BBa_K2549056) and ZF21.16 (Part:BBa_K2549046), from N terminal to C terminal. VP64 is a tetrameric VP16 transcription activator which shows ultrahigh transcription activation function. dNLS, which is short for destroyable nuclear localization sequence, is able to guide the fusion protein to be located to the nucleus without TEV protease. It is cleaved and unable to guide the fusion protein when TEV protease exist. When coexpressed with NLS-TEVp (Part:BBa_K2549041), it will be cleaved and the transcription activation function cannot be conducted. It is available to wire more complex binary logic gates including XOR gate and XNOR gate due to its wonderful feature.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 502
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Biology
Please visit http://2018.igem.org/Team:Fudan/Results and http://2018.igem.org/Team:Fudan/Measurement to check how we use this.
TEV protease-based transcription regulation or proteolysis-only activity
TEV protease is widely used in synthetic biology for its high cleavage specificity (targetting amino acids sequence ENLYFQG/S between QG or QS)[1] and cleaves and high efficiency (optimized by Kapust RB et al to remove autolysis)[2]. Wehr MC et al have demonstrated the TEV activity-dependent activation of several reporters[3]. In our system, we set the TEV protease specific cleavage site into the spacer region of the nuclear localization sequence[4], which is the critical design of our TEV protease-based complex logic gates.
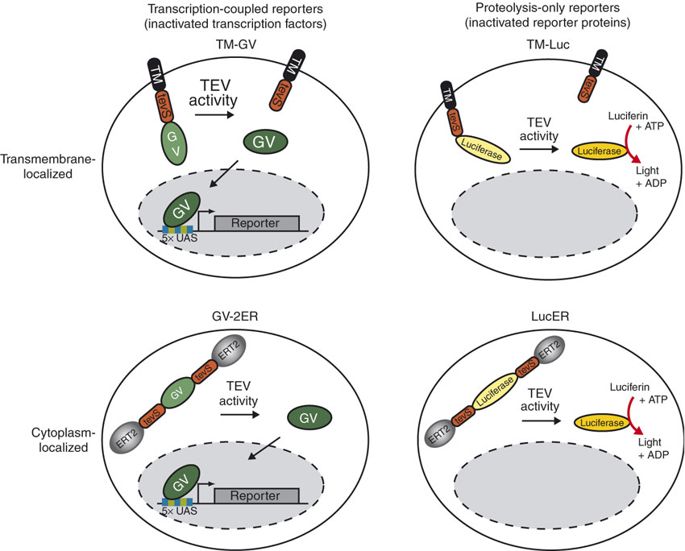
For more details about TEV protease, please refer to our TEVp (Part:BBa_K2549013).
References
- ↑ Release of proteins and peptides from fusion proteins using a recombinant plant virus proteinase. Parks TD, Leuther KK, Howard ED, Johnston SA, Dougherty WG. Anal Biochem, 1994 Feb;216(2):413-7 PMID: 8179197; DOI: 10.1006/abio.1994.1060
- ↑ Tobacco etch virus protease: mechanism of autolysis and rational design of stable mutants with wild-type catalytic proficiency. Kapust RB, Tözsér J, Fox JD, ..., Copeland TD, Waugh DS. Protein Eng, 2001 Dec;14(12):993-1000 PMID: 11809930
- ↑ Monitoring regulated protein-protein interactions using split TEV. Wehr MC, Laage R, Bolz U, ..., Nave KA, Rossner MJ. Nat Methods, 2006 Dec;3(12):985-93 PMID: 17072307; DOI: 10.1038/nmeth967
- ↑ Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Robbins J, Dilworth SM, Laskey RA, Dingwall C. Cell, 1991 Feb;64(3):615-23 PMID: 1991323
