Difference between revisions of "Part:BBa K2549040"
(→Biology) |
(→Our characterization) |
||
| (7 intermediate revisions by 2 users not shown) | |||
| Line 4: | Line 4: | ||
This part is one of the downstream elements of our amplifier. It is constructed by fusing KRAB ([[Part:BBa_K2549055]]), dNLS ([[Part:BBa_K2549056]]) and ZF21.16 ([[Part:BBa_K2549046]]), from N terminal to C terminal. KRAB is a strong transcription repressor. dNLS, which is short for destroyable nuclear localization sequence, is able to guide the fusion protein to be located to the nucleus without TEV protease. It is cleaved and unable to guide the fusion protein when TEV protease exist. Thus when coexpressed with NLS-TEVp ([[Part:BBa_K2549041]]), it is cleaved and the transcription repression function cannot be conducted. | This part is one of the downstream elements of our amplifier. It is constructed by fusing KRAB ([[Part:BBa_K2549055]]), dNLS ([[Part:BBa_K2549056]]) and ZF21.16 ([[Part:BBa_K2549046]]), from N terminal to C terminal. KRAB is a strong transcription repressor. dNLS, which is short for destroyable nuclear localization sequence, is able to guide the fusion protein to be located to the nucleus without TEV protease. It is cleaved and unable to guide the fusion protein when TEV protease exist. Thus when coexpressed with NLS-TEVp ([[Part:BBa_K2549041]]), it is cleaved and the transcription repression function cannot be conducted. | ||
| + | |||
| + | <!-- --> | ||
| + | <span class='h3bb'>Sequence and Features</span> | ||
| + | <partinfo>BBa_K2549040 SequenceAndFeatures</partinfo> | ||
| + | |||
<!-- Add more about the biology of this part here --> | <!-- Add more about the biology of this part here --> | ||
===Biology=== | ===Biology=== | ||
| + | =====Our characterization===== | ||
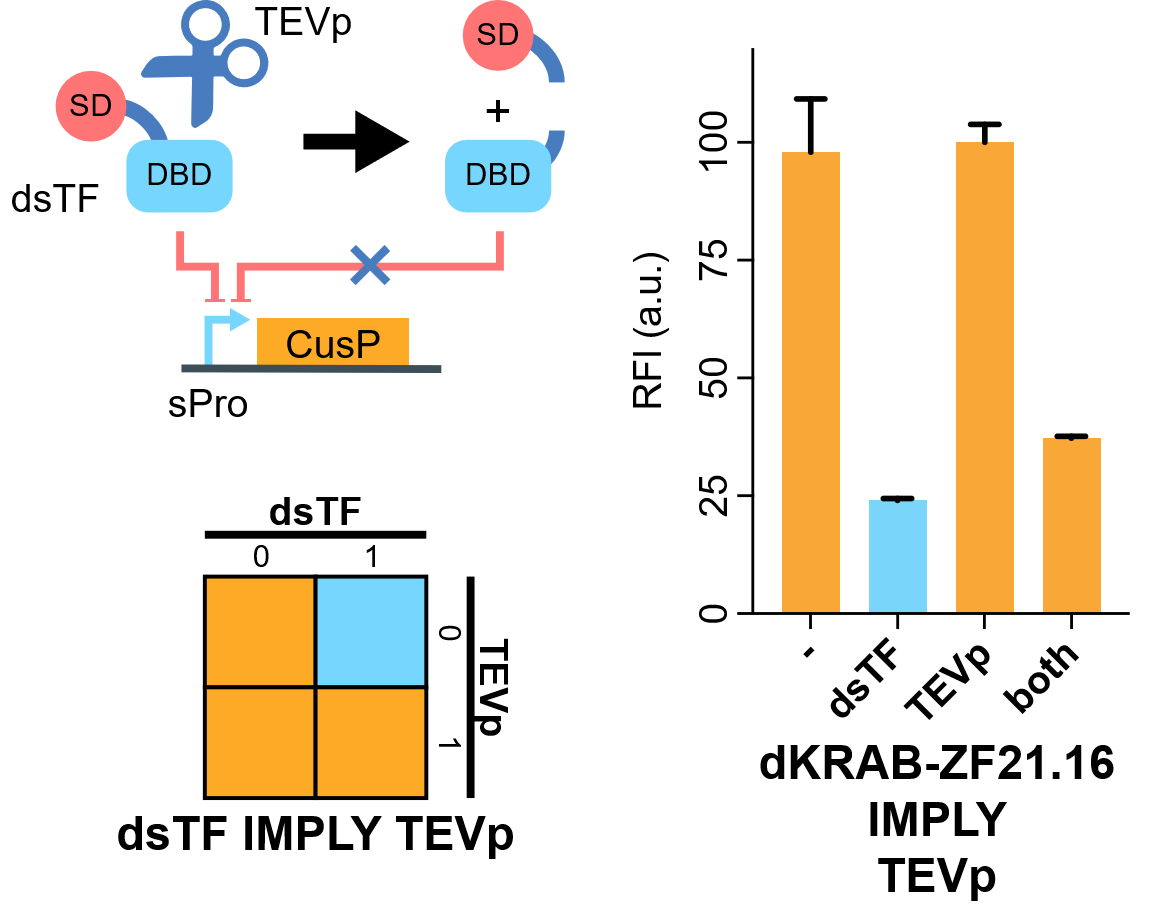
| + | [[File:IMPLY.png|none|410px|thumb|'''TEVp-based IMPLY gate.''' A degradable EGFP (d2EGFP) is produced downstream the promoter of the Combiner to indicate the output (CusP) strength. DBD, DNA binding domain which is zinc finger in our assay. SD, silencing-form transcriptional domain. RE, responsive elements. RFI, relative fluorescence intensity. More details please visit http://2018.igem.org/Team:Fudan/Results and http://2018.igem.org/Team:Fudan/Measurement .]] | ||
| + | |||
| + | We show that when KRAB-dNLS-ZF21.16 ([[Part:BBa_K2549040]]) exist while TEVp ([[Part:BBa_K2549041]]) did not, the expression of d2EGFP was repressed by KRAB. When TEVp and KRAB-dNLS-ZF21.16 both were present, TEVp cleaves dNLS and prevents KRAB functional, although the release from the suppression was not as strong as we hoped. NS3 cis-protease is on our list to try next<ref>Chemogenetic control of gene expression and cell signaling with antiviral drugs. Tague EP, Dotson HL, Tunney SN, Sloas DC, Ngo JT. Nat Methods, 2018 Jul;15(7):519-522 PMID: 29967495; DOI: 10.1038/s41592-018-0042-y</ref>. | ||
| + | |||
====TEV protease-based transcription regulation or proteolysis-only activity==== | ====TEV protease-based transcription regulation or proteolysis-only activity==== | ||
TEV protease is widely used in synthetic biology for its high cleavage specificity (targetting amino acids sequence ENLYFQG/S between QG or QS)<ref>Release of proteins and peptides from fusion proteins using a recombinant plant virus proteinase. Parks TD, Leuther KK, Howard ED, Johnston SA, Dougherty WG. Anal Biochem, 1994 Feb;216(2):413-7 PMID: 8179197; DOI: 10.1006/abio.1994.1060</ref> and cleaves and high efficiency (optimized by RB Kapust et al to remove autolysis)<ref>Tobacco etch virus protease: mechanism of autolysis and rational design of stable mutants with wild-type catalytic proficiency. Kapust RB, Tözsér J, Fox JD, ..., Copeland TD, Waugh DS. Protein Eng, 2001 Dec;14(12):993-1000 PMID: 11809930</ref>. Rossner MJ et al have demonstrated the TEV activity-dependent activation of several reporters<ref>Monitoring regulated protein-protein interactions using split TEV. Wehr MC, Laage R, Bolz U, ..., Nave KA, Rossner MJ. Nat Methods, 2006 Dec;3(12):985-93 PMID: 17072307; DOI: 10.1038/nmeth967</ref>. '''In our system, we set the TEV protease specific cleavage site into the spacer region of the nuclear localization sequence<ref>Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Robbins J, Dilworth SM, Laskey RA, Dingwall C. Cell, 1991 Feb;64(3):615-23 PMID: 1991323</ref>, which is the core design of the TEV protease-based complex logic gates.''' | TEV protease is widely used in synthetic biology for its high cleavage specificity (targetting amino acids sequence ENLYFQG/S between QG or QS)<ref>Release of proteins and peptides from fusion proteins using a recombinant plant virus proteinase. Parks TD, Leuther KK, Howard ED, Johnston SA, Dougherty WG. Anal Biochem, 1994 Feb;216(2):413-7 PMID: 8179197; DOI: 10.1006/abio.1994.1060</ref> and cleaves and high efficiency (optimized by RB Kapust et al to remove autolysis)<ref>Tobacco etch virus protease: mechanism of autolysis and rational design of stable mutants with wild-type catalytic proficiency. Kapust RB, Tözsér J, Fox JD, ..., Copeland TD, Waugh DS. Protein Eng, 2001 Dec;14(12):993-1000 PMID: 11809930</ref>. Rossner MJ et al have demonstrated the TEV activity-dependent activation of several reporters<ref>Monitoring regulated protein-protein interactions using split TEV. Wehr MC, Laage R, Bolz U, ..., Nave KA, Rossner MJ. Nat Methods, 2006 Dec;3(12):985-93 PMID: 17072307; DOI: 10.1038/nmeth967</ref>. '''In our system, we set the TEV protease specific cleavage site into the spacer region of the nuclear localization sequence<ref>Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Robbins J, Dilworth SM, Laskey RA, Dingwall C. Cell, 1991 Feb;64(3):615-23 PMID: 1991323</ref>, which is the core design of the TEV protease-based complex logic gates.''' | ||
| Line 14: | Line 24: | ||
For more details about TEV protease, please refer to our TEVp ([[Part:BBa_K2549013]]). | For more details about TEV protease, please refer to our TEVp ([[Part:BBa_K2549013]]). | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
Latest revision as of 21:34, 17 October 2018
KRAB-dNLS-ZF21.16
This part is one of the downstream elements of our amplifier. It is constructed by fusing KRAB (Part:BBa_K2549055), dNLS (Part:BBa_K2549056) and ZF21.16 (Part:BBa_K2549046), from N terminal to C terminal. KRAB is a strong transcription repressor. dNLS, which is short for destroyable nuclear localization sequence, is able to guide the fusion protein to be located to the nucleus without TEV protease. It is cleaved and unable to guide the fusion protein when TEV protease exist. Thus when coexpressed with NLS-TEVp (Part:BBa_K2549041), it is cleaved and the transcription repression function cannot be conducted.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 577
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Biology
Our characterization
We show that when KRAB-dNLS-ZF21.16 (Part:BBa_K2549040) exist while TEVp (Part:BBa_K2549041) did not, the expression of d2EGFP was repressed by KRAB. When TEVp and KRAB-dNLS-ZF21.16 both were present, TEVp cleaves dNLS and prevents KRAB functional, although the release from the suppression was not as strong as we hoped. NS3 cis-protease is on our list to try next[1].
TEV protease-based transcription regulation or proteolysis-only activity
TEV protease is widely used in synthetic biology for its high cleavage specificity (targetting amino acids sequence ENLYFQG/S between QG or QS)[2] and cleaves and high efficiency (optimized by RB Kapust et al to remove autolysis)[3]. Rossner MJ et al have demonstrated the TEV activity-dependent activation of several reporters[4]. In our system, we set the TEV protease specific cleavage site into the spacer region of the nuclear localization sequence[5], which is the core design of the TEV protease-based complex logic gates.
For more details about TEV protease, please refer to our TEVp (Part:BBa_K2549013).
References
- ↑ Chemogenetic control of gene expression and cell signaling with antiviral drugs. Tague EP, Dotson HL, Tunney SN, Sloas DC, Ngo JT. Nat Methods, 2018 Jul;15(7):519-522 PMID: 29967495; DOI: 10.1038/s41592-018-0042-y
- ↑ Release of proteins and peptides from fusion proteins using a recombinant plant virus proteinase. Parks TD, Leuther KK, Howard ED, Johnston SA, Dougherty WG. Anal Biochem, 1994 Feb;216(2):413-7 PMID: 8179197; DOI: 10.1006/abio.1994.1060
- ↑ Tobacco etch virus protease: mechanism of autolysis and rational design of stable mutants with wild-type catalytic proficiency. Kapust RB, Tözsér J, Fox JD, ..., Copeland TD, Waugh DS. Protein Eng, 2001 Dec;14(12):993-1000 PMID: 11809930
- ↑ Monitoring regulated protein-protein interactions using split TEV. Wehr MC, Laage R, Bolz U, ..., Nave KA, Rossner MJ. Nat Methods, 2006 Dec;3(12):985-93 PMID: 17072307; DOI: 10.1038/nmeth967
- ↑ Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Robbins J, Dilworth SM, Laskey RA, Dingwall C. Cell, 1991 Feb;64(3):615-23 PMID: 1991323
