Difference between revisions of "Part:BBa K592009:Experience"
| Line 273: | Line 273: | ||
<h1>Austin_UTexas 2017 Experience</h1> | <h1>Austin_UTexas 2017 Experience</h1> | ||
<br> | <br> | ||
| − | Due to the instability of the original blue chromoprotein sequence, cells expressing the blue chromoprotein have difficulty retaining the blue phenotype. In order to combat this metabolic burden, a technique called codon optimization was used to improve the function of this part as a reliable and stable color reporter. Codon optimization involves exchanging certain codons for ones that have known to be more translationally efficient in a certain species while retaining the same sequence of amino acids. Making the translation process more efficient, in turn, lowers the metabolic burden in the cells that the blue chromoprotein gene is present in. | + | Due to the observed instability of the original blue chromoprotein sequence, cells expressing the blue chromoprotein have difficulty retaining the blue phenotype. In order to combat this metabolic burden, a technique called codon optimization was used to improve the function of this part as a reliable and stable color reporter. Codon optimization involves exchanging certain codons for ones that have known to be more translationally efficient in a certain species while retaining the same sequence of amino acids. Making the translation process more efficient, in turn, lowers the metabolic burden in the cells that the blue chromoprotein gene is present in. |
To create the codon-optimized blue chromoprotein BioBrick part, a digestion was performed on the BBa_K608002 vector (within the PSB1C3 backbone) and the codon-optimized blue chromoprotein, which was ordered as a gBlock from the Integrated DNA Technologies (IDT) website. Upon digesting each of these parts and conducting a gel extraction, a ligation reaction was used to join the codon-optimized blue chromoprotein with the K608002 vector. Following the purification of the ligation, a transformation was done via electroporation which yielded one phentotypically blue colony, shown below in Figure 1. This colony was then inoculated into liquid media to make an overnight culture, shown in Figure 2, which maintained a blue phenotype. | To create the codon-optimized blue chromoprotein BioBrick part, a digestion was performed on the BBa_K608002 vector (within the PSB1C3 backbone) and the codon-optimized blue chromoprotein, which was ordered as a gBlock from the Integrated DNA Technologies (IDT) website. Upon digesting each of these parts and conducting a gel extraction, a ligation reaction was used to join the codon-optimized blue chromoprotein with the K608002 vector. Following the purification of the ligation, a transformation was done via electroporation which yielded one phentotypically blue colony, shown below in Figure 1. This colony was then inoculated into liquid media to make an overnight culture, shown in Figure 2, which maintained a blue phenotype. | ||
<html> | <html> | ||
Revision as of 00:49, 2 November 2017
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_K592009
User review of SHSBNU_China
Figure 1 TtrS/R system and ThsS/R system ThsS (BBa_K2507000) and ThsR (BBa_K2507001), both codon-optimized for E. coli, are two basic parts which belong to the two-component system from the marine bacterium Shewanella halifaxensis. By linking thsR with chromoprotein genes (BBa_K2507009, BBa_K2507010, BBa_K2507011) or the violacein producing operon vioABDE (BBa_K2507012), this system can respond to thiosulfate by producing a signal visible to the naked eye, such as chromoproteins (spisPink-pink chromoprotein, gfasPurple-purple chromoprotein, amilCP-blue chromoprotein) or a dark-green small-molecule pigment (protoviolaceinic acid). E. coli-codon-optimized TtrS(BBa_K2507002) and TtrR (BBa_K2507003) are two basic parts which are derived from the two-component system of the marine bacterium Shewanella baltica.
By linking ttrR with chromoprotein genes (BBa_K2507009, BBa_K2507010, BBa_K2507011) or the violacein producing operon vioABDE (BBa_K2507012), this system can respond to thiosulfate by producing a signal visible to the naked eye such as chromoproteins (spisPink-pink chromoprotein, gfasPurple-purple chromoprotein, amilCP-blue chromoprotein) or a dark-green small-molecule pigment (protoviolaceinic acid).
Figure 2. Characterization of the ThsS/R and TtrS/R system by observing the chromprotein expression levels. We added 1mM, 0.1mM, 0.01mM and 0 Na2S2O3 to ThsS/R system and added 2.5mM, 1mM, 0.1mM and 0 Na2S4O6·2H2O to ThsS/R system. The results demonstrate there is an obvious response in the ThsS/R system. In a. gfasPurple system, the response curve is obvious, and b. spisPink & c. amilCP with rather heavy leaky expression without inducer. d.e.f. However, in TtrS/R system there is no clear result.
Figure 3. Characterization of the ThsS/R and TtrS/R system by observing the protoviocaceinic acid( dark-green pigment). We added 1mM, 0.1mM, 0.01mM and 0 Na2S2O3 to ThsS/R system and added 2.5mM, 1mM, 0.1mM and 0 Na2S4O6·2H2O to ThsS/R system. The results demonstrate there is an obvious response in the ThsS/R system with rather heavy leaky expression without inducer. And the Ths/R+protoviolaceinic acid works rather well!
User Reviews
UNIQ0b0ee8b00fce9161-partinfo-00000000-QINU
UNIQ0b0ee8b00fce9161-partinfo-00000001-QINU
UNIQ0b0ee8b00fce9161-partinfo-00000002-QINU
|
•••••
iGEM Sydney Australia |
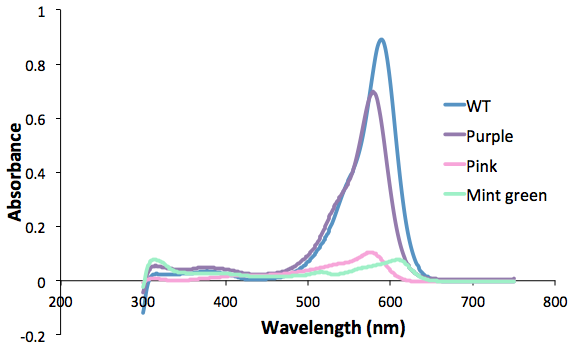
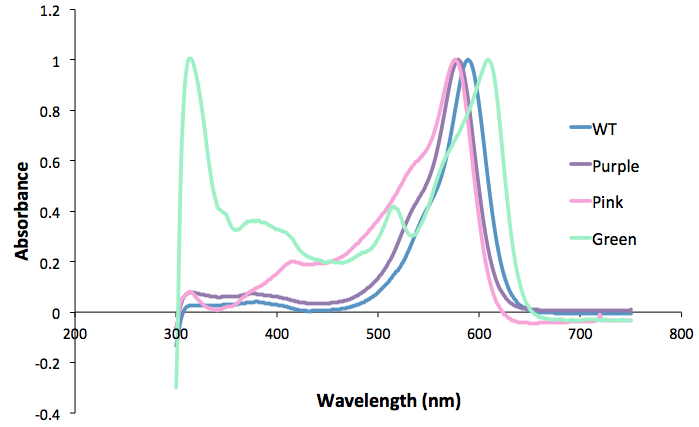
Figure 1. Purple, pink and green mutants compared to the wild type.
|
|
•••••
iGEM Uppsala 2013 |
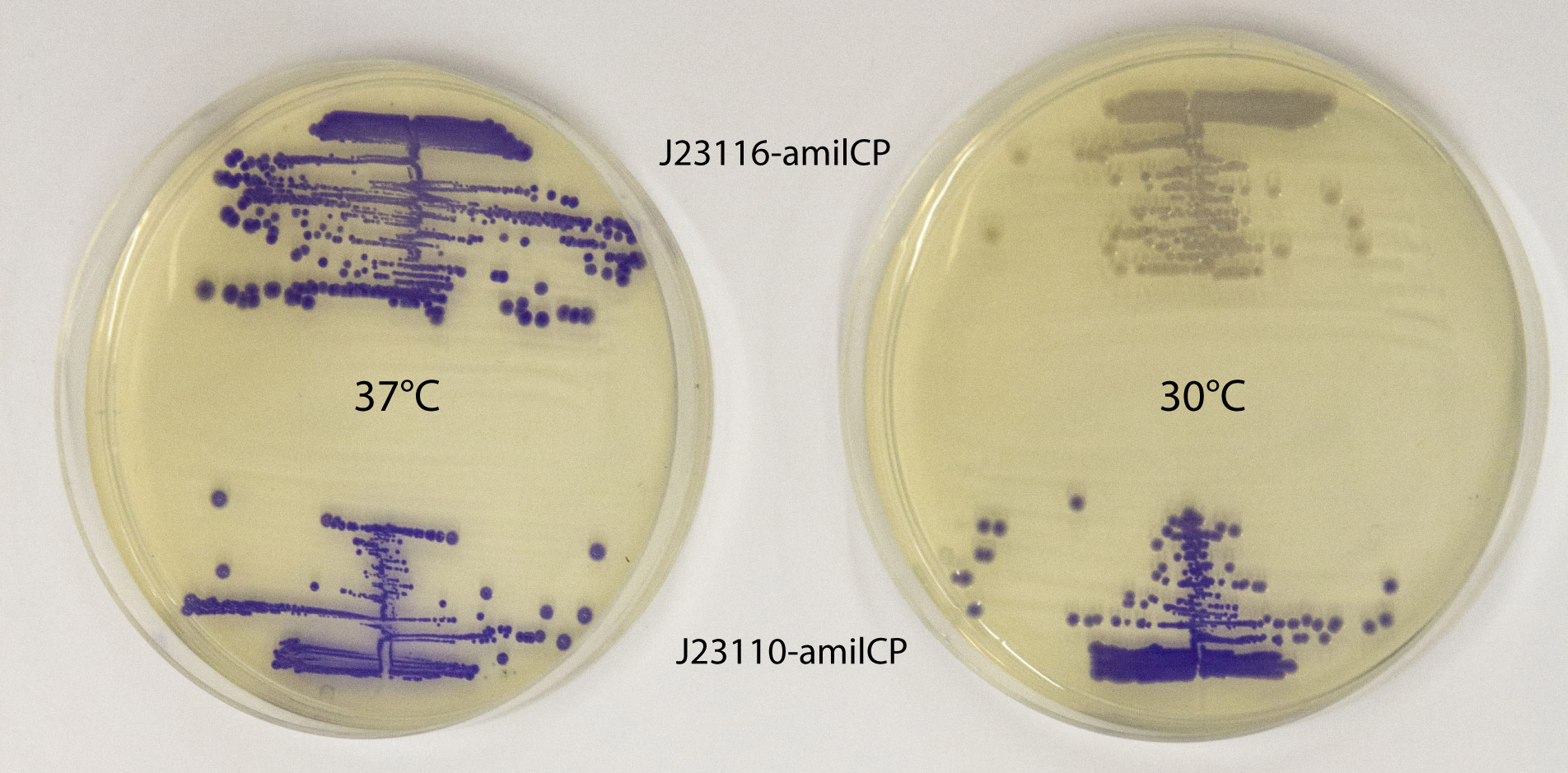
This part works well as a reporter. As expected, different levels of expression of amilCP in E. coli MG1655 generates different intensities in color. amilCP expressed from the high copy plasmid pSB1C3 by weak promoter J23116 (top part of plates) and strong promoter J23110 (bottom part of plates) at 37 °C (left plate) and 30 °C (right plate) respectively. After incubation at 30 °C a clear difference in color intensity can be seen between the expression from the two different promoters, while after incubation at 37 °C the difference in intensity is much less pronounced. This indicates that expression from the promoters are temperature dependent, and it shows that the quantifiable range of the chromoproteins is somewhat limited. Plates were incubated for 23 h at 37 °C or 30 °C followed by 20 h at 22 °C. |
UNIQ0b0ee8b00fce9161-partinfo-00000005-QINU
|
•••••
Paris_Saclay 2013 |
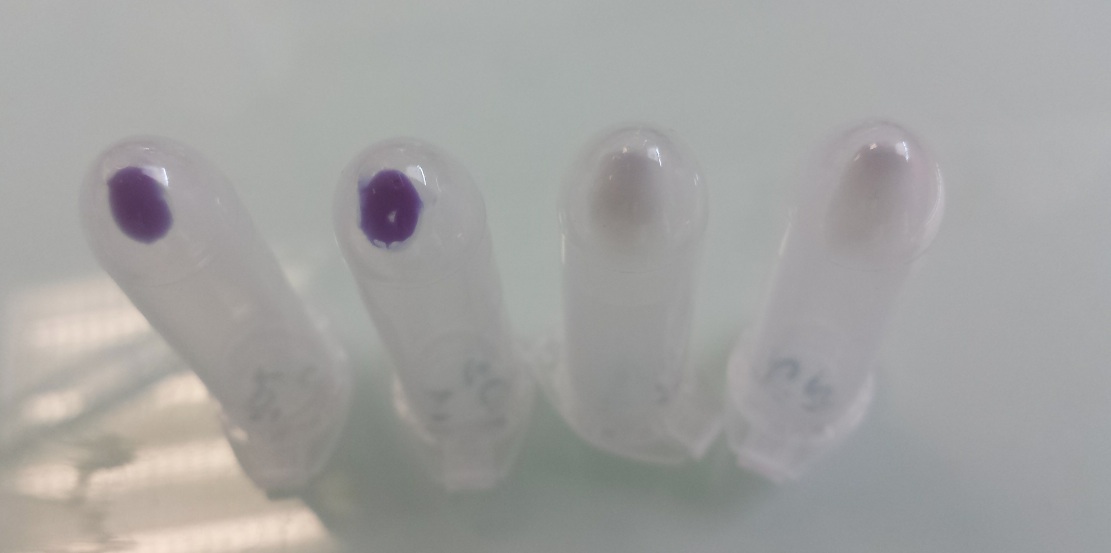
Our team improved the biobrick BBa_K592009 by assembly of biobricks B0034, BBa_K592009 and B0015 to construct a composite part including a ribosome binding site, full length of amilCP gene and double terminator. We used it as one of our reporter gene in Escherichia coli to controlled activator or repressor activity of our promotor under aerobic or anaerobic conditions (Pndh*, PnirB, PnarG, PnarK, PbphR1, PbphR2). Construction : Pndh* (repressor) + BBa_K1155003 :
Tubes 1 and 2 : cellular pallet of cultures grown in aerobic conditions Tubes 3 and 4 : cellular pallet of cultures grown in anaerobic conditions |
UNIQ0b0ee8b00fce9161-partinfo-00000007-QINU
UNIQ0b0ee8b00fce9161-partinfo-00000008-QINU
UNIQ0b0ee8b00fce9161-partinfo-00000009-QINU
|
•••••
iGEM Michigan 2013 |
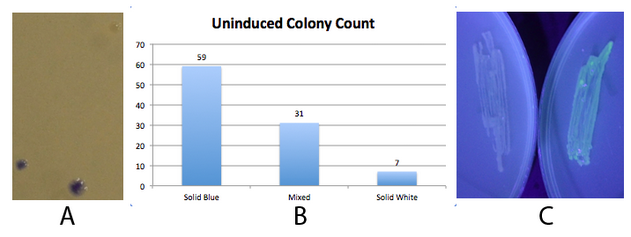
The 2013 Michigan igem team successfully used this part to produce amilCP. Fig 2. - An inducible fim transcriptor system changes states and produces protein output NEB 10-beta E. coli, which lack the native fim switch (fimS) and known fim recombinases, were co-transformed with K1077007(amilCP j23100 fim switch) or K1077003(GFP j23100 fim switch), and K1077002 (aTc inducible fimE, HSL inducible hbiF) and plated on to LB plates with or without inducer. A) Close up of 3 colonies on a plate containing K1077007 and K1077002 co transformants. Three distinct phenotypes were observed: Solid Blue colonies(bottom left), Mixed colonies that had distinct white and blue regions(bottom middle), and Solid White colonies(top right). Therefore, the uninduced fim transcriptor in the context of K1077002 is subject to leaky fimE and/or hbiF activity. B) Quantification of the phenotypes observed on the plate in A. C) When induced with 4.32µM aTc and grown overnight (left) , no GFP is produced as expected due to induced fimE turning the switch OFF. When induced with 1µM HSL and grown overnight (right), GFP is produced as expected due to induced hbiF turning the switch ON. |
|
•••••
Immudzen |
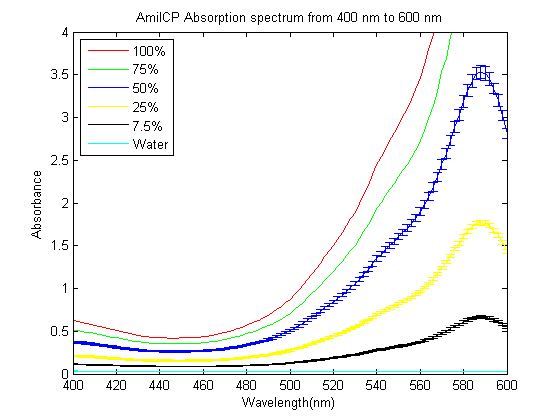
As part of the 2013 CU Boulder project we worked on separating RFP from AmilCP and we measured the spectrum of AmilCP from 400 nm to 600 nm. We also found that when running on an agarose gel RFP will run down on the gel while AmilCP runs up on the gel. |
|
•••••
iGEM 2013 Hong_Kong_HKUST Trevor Y.H. HO |
Error creating thumbnail: Invalid thumbnail parameters This part was employed in testing our Gibson Assembly protocol. In the experiment design, the mRFP coding DNA sequence in J04450 was replaced with that of amilCP. The resulting plate from transformation of the post-reaction mixture is shown. The color of the blue chromoprotein was clearly distinguished from that of mRFP, which aided in my evaluation of the performance of Gibson Assembly. |
|
BBa_K592009 iGEM Groningen 2012 |
Our team has managed to couple this biobrick part with our promoters: alsT, fnr, and sboA. The cloning was done in BBa_K818000 (plasmid backbone for B. subtilis, engineered by team Groningen 2012), to allow color expression in B. subtilis. We utilized a strong RBS BBa_B0034 for pigment expression in E. coli and B. subtilis. The purple/blue colour was strongly visible in E.coli without any induction, while the expression in B. subtilis was more subtle (B. subtilis colony looks slightly blue on the plate agar). After induction of the promoter before AmilCP, B. subtilis also turned clearly purple/blue. Please have a look at the page of BBa_K818400 for more information. |
|
No review score entered. User:agynna |
iGEM Team Uppsala University 2012 We have observed that expression of amilCP confers a noticeable fitness cost in E coli. This is surprising, given that such behaviour is not seen in other homologous chromoproteins and fluorescent proteins. We thus recommend using the new aeBlue (BBa_K864401) chromoprotein for blue color expression, which also has a more clear blue color. !!!!!!! |
|
BBa_K592009 iGEM DTU-Denmark 2017 |
The DTU-Denmark 2017 team has added a 6 x his-tag to the C-terminal end of the amilCP protein, to ensure easy purification of amilCP using affinity chromatography. The his-tagged amilCP was submitted to the parts registry as BBa_K2355002. The recombinant amilCP with his-tag was expressed in E.coli, and the and the His-tagged amilCP was purified using a Ni-NTA spin colomn. Compared to a control with expressed amilCP from BBa_K592009, the his-tagged amilCP had a 2-4 fold increase in amilCP concentration following the his-tag purification. Take a look at the entry of BBa_K2355002 for more information. |
BNU-China 2017 Experience
Team UCLA in 2015 iGEM used honeybee silk protein 3 (part number: BBa_K1763000) in their project, which can serve the purpose of being the substitution of spider thread and natural silk. Although it is not as strong as other kind of silk protein, the honeybee silk protein has some advantages compared to spider thread and natural silk: the gene of honeybee silk protein is smaller, with no duplicated sequences, and easier to be synthesized and modified by molecular biology technology.
However, the silk protein produced from UCLA has no color, thus with less aesthetic characteristic and uneasy to observe directly. In order to observe the produced honeybee silk protein more directly, we add a blue chromoprotein, amilCP (part number: BBa_K592009) , to the end of the silk protein. AmilCP is a blue chromoprotein comes from coral, which has a strong blue after expressing. The color has the biggest absorption peak around 588nm, which can be observed by naked eyes directly without any equipment.
We designed 2 pairs of primers for the genes of honeybee silk protein and blue chromoprotein by molecular biology technology. (There is a GGGGS linker in the middle of two fragments. ) Then we use infusion technology to insert two fragments after T7 promoter of pET21a(+) vector. The verification by PCR is showed in Figure 1. After then we introduced the recombinant plasmid into E.coli BL21(DE3). In 48 hours, there is blue chromoprotein expressed in bacterium solution, which can be observed by naked eyes (Figure 2). Therefore, the improvement of honeybee silk protein is practical and valid.


The result of the experiment is showed in the following figures. Figure 3 showed the different colors of E.coli DL21 with different expression levels after centrifugation. There are largely expression group, slightly expression group and control group from left to right. The samples are investigated by SDS-PAGE after crushing the bacteria of three groups by high temperature. As it is showed in Figure 4 , after dyed by coomassie brilliant blue, there is a large number of protein around expected 56-57kD from largely expression group, a small number of protein from slightly expression group, and almost no significant expression from control group. The result of SDS-PAGE shows that the improved honeybee silk protein can be observed by naked eyes, and the different color shades of blue can reflect the expression level of the protein.


By infusing chromoprotein with different colors after the honeybee silk protein, we can produce silk protein with different color, which will allow us to obtain the expression time, expression level and expression efficiency of the silk protein, therefore control the production of the honeybee silk protein efficiently. Not only can we make the silk protein more aesthetic, but also we can apply the silk protein into more areas widely, such as health and medicine, textile industry and biomaterial.
In this way we creat a new part: Honeybee-blue with amilCP protein (BBa_K2220028)
Austin_UTexas 2017 Experience
Due to the observed instability of the original blue chromoprotein sequence, cells expressing the blue chromoprotein have difficulty retaining the blue phenotype. In order to combat this metabolic burden, a technique called codon optimization was used to improve the function of this part as a reliable and stable color reporter. Codon optimization involves exchanging certain codons for ones that have known to be more translationally efficient in a certain species while retaining the same sequence of amino acids. Making the translation process more efficient, in turn, lowers the metabolic burden in the cells that the blue chromoprotein gene is present in.
To create the codon-optimized blue chromoprotein BioBrick part, a digestion was performed on the BBa_K608002 vector (within the PSB1C3 backbone) and the codon-optimized blue chromoprotein, which was ordered as a gBlock from the Integrated DNA Technologies (IDT) website. Upon digesting each of these parts and conducting a gel extraction, a ligation reaction was used to join the codon-optimized blue chromoprotein with the K608002 vector. Following the purification of the ligation, a transformation was done via electroporation which yielded one phentotypically blue colony, shown below in Figure 1. This colony was then inoculated into liquid media to make an overnight culture, shown in Figure 2, which maintained a blue phenotype.