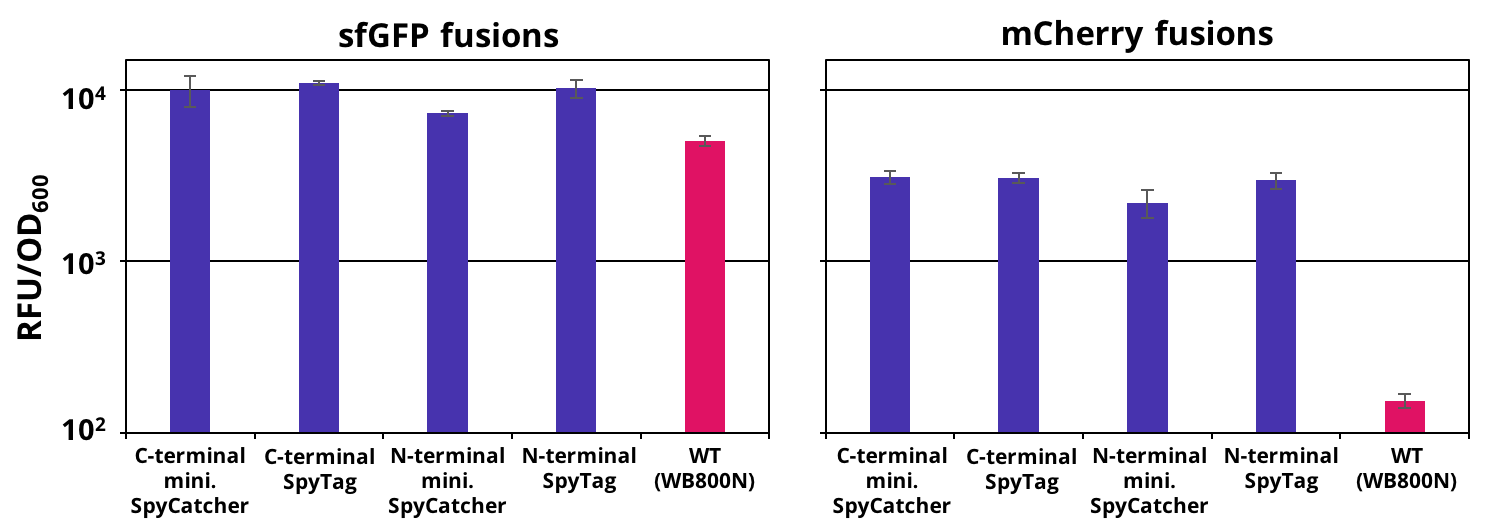Difference between revisions of "Part:BBa K2273035"
| Line 41: | Line 41: | ||
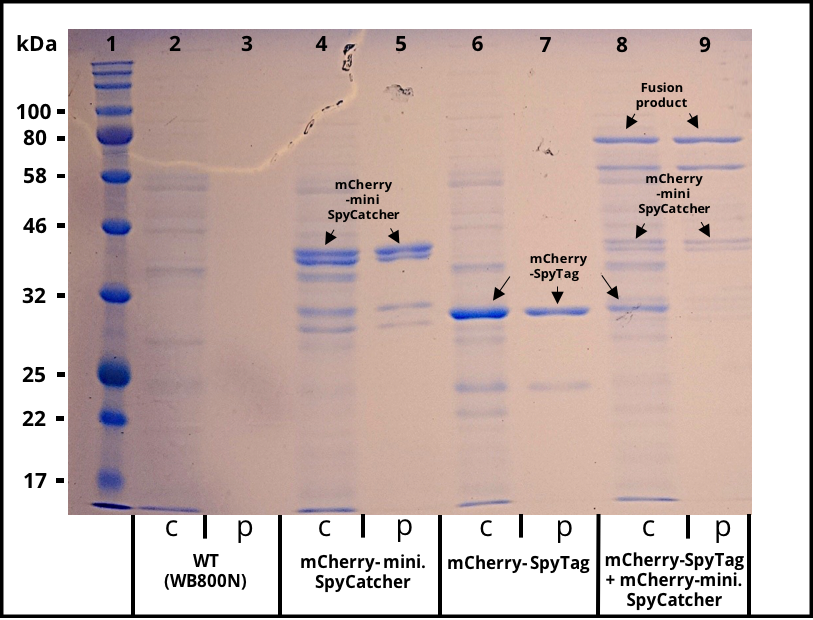
| − | The successful secretion could be proven with a fluorescence assay using the supernatants of <i>B. subtilis </i> (Figure 2 ). The functionality of the SpyTag/SpyCatcher was proven via SDS-PAGE, using the supernatants (Figure 3). | + | The successful secretion could be proven with a fluorescence assay using the supernatants of <i>B. subtilis </i> (Figure 2). The functionality of the SpyTag/SpyCatcher was proven via SDS-PAGE, using the supernatants (Figure 3). |
[[File:T--TU Dresden--secretion--Figure1.png|thumb|right|450px|'''Figure 2:''': Endpoint measurement of the fluorescence from supernatants carrying our constructs and the wild type. Expression of the single copy mCherry or sfGFP fusion SpyTag/SpyCather constructs (purple) was induced with 1% xylose and the supernatants were harvested after 16 h of incubation. Wild type supernatant is shown as a control (pink). Excitation wavelength for sfGFP was set to 480 nm and emission was recorded at 510 nm and for mCherry excitation wavelength was set to 585 nm and emission was recorded at 615 nm . The fluorescence was normalized by the optical density (OD600). Graph shows mean values and standard deviations of at least two biological and three technical replicates.]] | [[File:T--TU Dresden--secretion--Figure1.png|thumb|right|450px|'''Figure 2:''': Endpoint measurement of the fluorescence from supernatants carrying our constructs and the wild type. Expression of the single copy mCherry or sfGFP fusion SpyTag/SpyCather constructs (purple) was induced with 1% xylose and the supernatants were harvested after 16 h of incubation. Wild type supernatant is shown as a control (pink). Excitation wavelength for sfGFP was set to 480 nm and emission was recorded at 510 nm and for mCherry excitation wavelength was set to 585 nm and emission was recorded at 615 nm . The fluorescence was normalized by the optical density (OD600). Graph shows mean values and standard deviations of at least two biological and three technical replicates.]] | ||
Revision as of 15:05, 1 November 2017
| Part Information | |
|---|---|
| RFC standard | RFC 25 |
| Fused Tag | BBa_K2273016: Spytag His-tagged |
| Fluorescent Protein | BBa_K2273034: mCherry |
| Submitted by | [http://2017.igem.org/Team:TU_Dresden TU Dresden] |
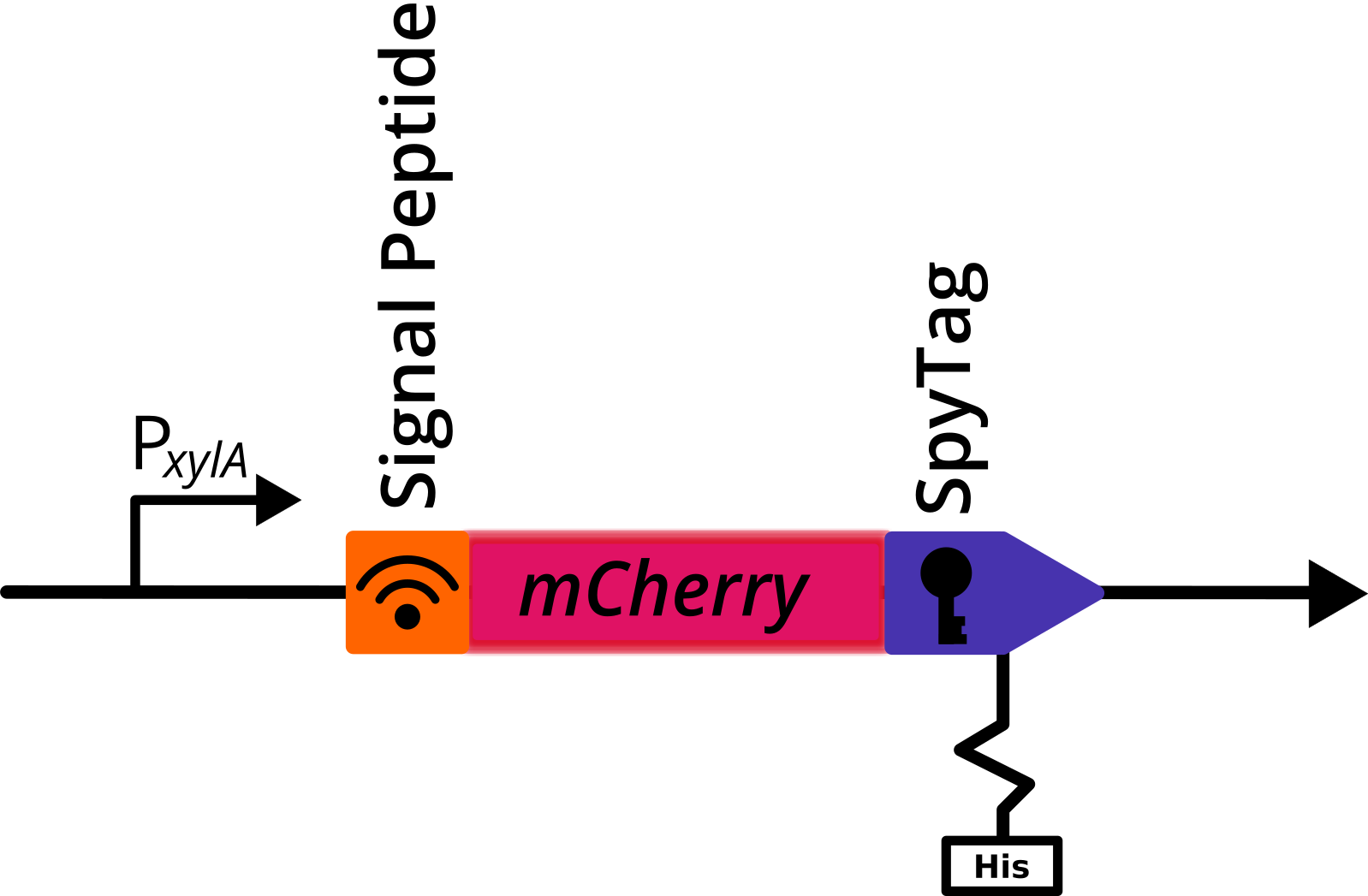
mCherry with C-Terminal SpyTag and His Tag
This composite part was used for evalutaion in the [http://2017.igem.org/Team:TU_Dresden/Project/Secretion secretion project] of 2017 TU_Dresden iGEM [http://2017.igem.org/Team:TU_Dresden team]. It codes for a fluorescent reporter protein (mCherry) and a functional tag (SpyTag His-tagged), mediating covalent bonding with it tag partner (SpyCatcher). It is optimized for usage in Bacillus subtilis.
Design
A signal peptide (AmyE SP) was fused n-terminally to this construct to induce secretion.
Application
The successful secretion could be proven with a fluorescence assay using the supernatants of B. subtilis (Figure 2). The functionality of the SpyTag/SpyCatcher was proven via SDS-PAGE, using the supernatants (Figure 3).