Difference between revisions of "Part:BBa K2446036"
| (3 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K2446036 short</partinfo> | <partinfo>BBa_K2446036 short</partinfo> | ||
| + | <div> | ||
| + | <B> A improving of <partinfo>BBa_K511003</partinfo> MIT_2011</B> | ||
| + | </div> | ||
| + | |||
| + | |||
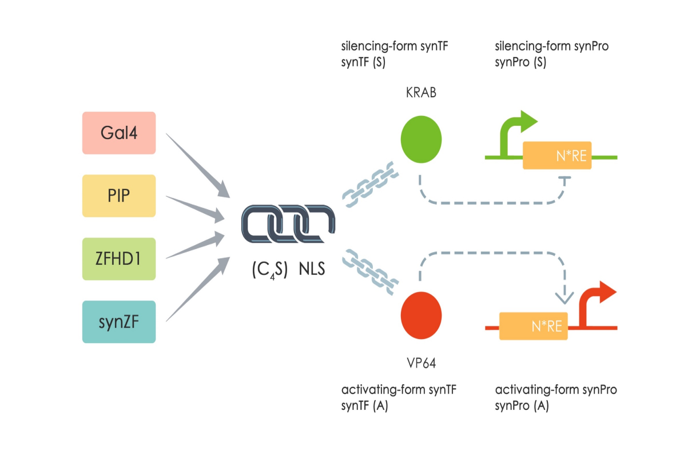
| + | This part (Sv40 UAS ) is a mammalian synthetic promoter (SynPro) based on Sv40 promoter (<partinfo>BBa_K2446052</partinfo>) and 4*GAL4 binding sites from UAS-Gal4 Promoter MammoBlock(<partinfo>BBa_K511003</partinfo> MIT2011) . We inset the 4*GAL4 binding sites behind the Sv40 promoter. Sv40 promoter is a constitutive expression promoter from the simian vacuolating virus 40. In this way, we can use our mammalian synthetic transcription factors (SynTFs) which is a fusing protein GAL4_DBD_(G4S) linker_NLS_KRAB. The GAL4DBD can bind to the 4*GAL4 binding sites specifically[[#References|[1]]] and the KRAB domain can repress the expression of Sv40 UAS promoter [[#References|[2]]]. The corresponding SynTF of Sv40-UAS is ZF_GAl4_KRAB (<partinfo>BBa_K2446037</partinfo>). | ||
| + | |||
| + | [[file:T--Fudan--design.png|500px|middle|'''Figure 1''': The design of SynTF-SynPros.]] | ||
| + | <div> | ||
| + | <B> Figure 1: The design of SynTF-SynPros.</B> | ||
| + | </div> | ||
| + | |||
| + | |||
| + | The information of other SynTF-SynPros is showed in the table below. | ||
| + | <table border="2" width="800px" > | ||
| + | <tr> | ||
| + | <th>SynTFs </th> | ||
| + | <th>SynPros</th> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Gal4-KRAB(TF-KRAB-1) <partinfo>BBa_K2446037</partinfo></td> | ||
| + | <td>Sv40-UAS(Sv40-UAS) <partinfo>BBa_K2446036</partinfo></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>ZF_PIP_KRAB(TF-KRAB-2) <partinfo>BBa_K2446045</partinfo></td> | ||
| + | <td>SV40_2/4/8_PIP <partinfo>BBa_K2446033</partinfo>/<partinfo>BBa_K2446034</partinfo>/<partinfo>BBa_K2446035</partinfo></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>ZF_21-16KRAB(TF-KRAB-3) <partinfo>BBa_K2446039 </partinfo></td> | ||
| + | <td>SV40_8_ZF_21-16 <partinfo>BBa_K2446030</partinfo></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>ZF_42-10_KRAB(TF-KRAB-4) <partinfo>BBa_K2446040</partinfo></td> | ||
| + | <td>SV40_8_ZF_42-10 <partinfo>BBa_K2446025</partinfo></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>ZF_43-8_KRAB(TF-KRAB-5) <partinfo>BBa_K2446041</partinfo></td> | ||
| + | <td>SV40_2/4/8_ZF_43-8 <partinfo>BBa_K2446026</partinfo>/<partinfo>BBa_K2446027</partinfo>/<partinfo>BBa_K2446028</partinfo></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>ZF_54-8_KRAB(TF-KRAB-6) <partinfo>BBa_K2446042</partinfo></td> | ||
| + | <td>SV40_8_ZF_54-8 <partinfo>BBa_K2446029</partinfo></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>ZFHD1_KRAB(TF-KRAB-7) <partinfo>BBa_K2446043</partinfo></td> | ||
| + | <td>SV40_4_ZFHD1 <partinfo>BBa_K2446032</partinfo></td> | ||
| + | </tr> | ||
| + | </table> | ||
| − | |||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
| Line 17: | Line 64: | ||
<partinfo>BBa_K2446036 parameters</partinfo> | <partinfo>BBa_K2446036 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
| + | ==Experiments== | ||
| + | ===SynTF-SynPro Pairs=== | ||
| + | |||
| + | <div> | ||
| + | |||
| + | [[file: T--Fudan--TF1c.png|500px|middle|'''Figure 1''': the testing circuits of GAL4-KRAB& Sv40 UAS pair]] | ||
| + | </div> | ||
| + | <div> | ||
| + | <B>Figure 1: the testing circuits of GAL4-KRAB& Sv40 UAS pair </B> | ||
| + | |||
| + | </div> | ||
| + | <div> | ||
| + | |||
| + | To make sure the SynTF-SynPro pairs work in mammalian cells, we use the circuits above to test if the Gal4-KRAB can repress the expression of Sv40-UAS indeed. Gal4-KRAB is linked to the C terminal of EGFP by the link of P2A. And mCherry expressions is controlled by corresponding SynPro (Sv40 UAS). These circuits are both inserted in to the mammalian expression vactor pML2. We transfect pML2-Sv40-UAS into Hela cells and measure the fluorescence intensity of mCherry by flow cytometer to get the basic expression intensity of Sv40-UAS. We also co-transfect the pML2-GAL4-KRAB with pML2-Sv40-UAS into Hela cells at the same time. Then measure the fluorescence intensity of mCherry again to get the expression intensity of Sv40-UAS influenced by GAL4-KRAB. The results of the experiment is showed below. The SynTF GAL4 can silence the expression of the SynPro Sv40-UAS in 25 folds. | ||
| + | |||
| + | <div> | ||
| + | |||
| + | [[file: T--Fudan--gal4.png|500px|'''Figure 2''':The results of GAL4-KRAB&SV40 testing: (A) The red points is the cells before co-transfecting GAL4-KRAB and the blue points is the cells after co-transfecting GAL4-KRAB. It’s easy to see that the red points depart from the diagonal and higher than the blue pints. So the expression of mCherry silenced after the expression of GAL4-KRAB;(B) The red area is the fluorescence intensity of mCherry before co-transfecting GAL4-KRAB and the blue area is the intensity after co-transfecting GAL4-KRAB.(C) The statistical result of all of the SynTFs-SynPros pairs: GAL4-KRAB can silence the expression intensity of Sv40-UAS in 25 folds]] | ||
| + | </div> | ||
| + | <div> | ||
| + | <B>Figure 2:The results of GAL4-KRAB&SV40 testing: </B>(A) The red points is the cells before co-transfecting GAL4-KRAB and the blue points is the cells after co-transfecting GAL4-KRAB. It’s easy to see that the red points depart from the diagonal and higher than the blue pints. So the expression of mCherry silenced after the expression of GAL4-KRAB;(B) The red area is the fluorescence intensity of mCherry before co-transfecting GAL4-KRAB and the blue area is the intensity after co-transfecting GAL4-KRAB.(C) The statistical result of all of the SynTFs-SynPros pairs: GAL4-KRAB can silence the expression intensity of Sv40-UAS in 25 folds | ||
| + | |||
| + | </div> | ||
| + | |||
| + | ===SynTF-SynPro Orthogonality=== | ||
| + | <div> | ||
| + | |||
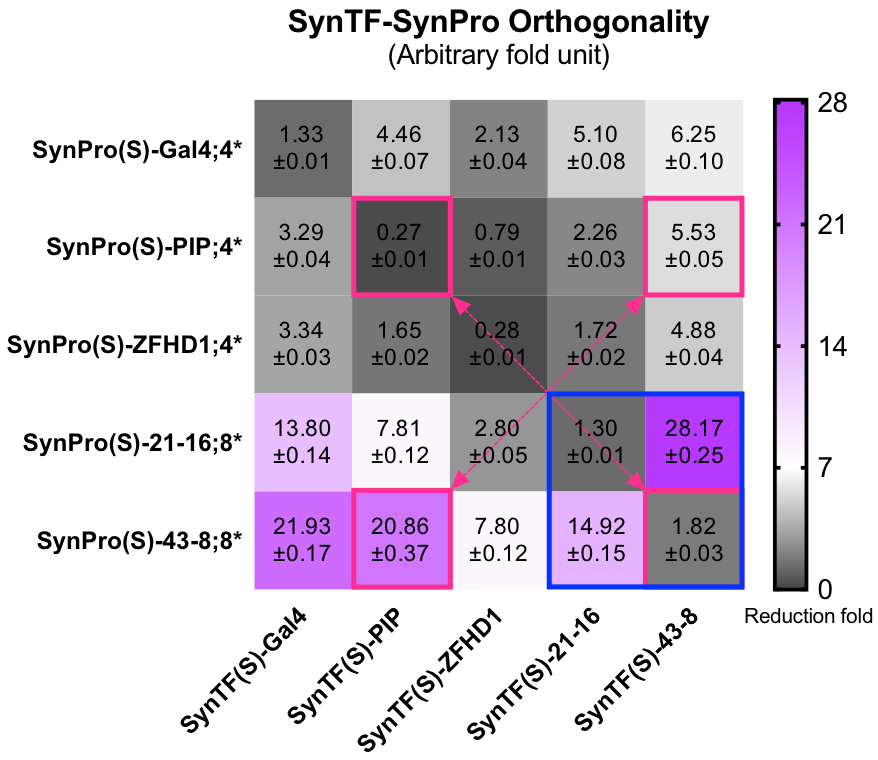
| + | To construct our [http://2017.igem.org/Team:Fudan/Model/GTN| Strip module], more than one SynTF-SynPro pairs would be applied. Thus, the interaction between the pairs would influence or ruin our construction. We did massive orthogonality experiments to avoid that. We observed all of the 5 pairs were actually orthogonal, as you could see the grids on the diagonal were always the darkest. The three DBDs commonly used in previous works were didn’t let us down. However, the expression level of these RE loaded SynPros were relative low compared to SynPro(S)-ZF serials. As the blue rectangle in the lower right corner of the orthogonality may showed the SynPro(S)-ZF has high basic expression with unpaired SynTFs, but could be silenced to the similar fold of commonly used DBDs corresponding SynPros. The SynPro(S)-ZF was likely won’t be target by other unpaired DBD, hence the purple appeared on the bottom rows. | ||
| + | |||
| + | </div> | ||
| + | <div> | ||
| + | |||
| + | [[file: T--Fudan--Orthogonality.png|500px|'''Figure 3''': the SynTF-SynPro pairs’ Orthogonality. Grids in blue rectangle showed that SynTF-SynPro pairs constructed by using SynZF as DBD with well orthogonality. Grids in pink rectangles replaced our favorite SynTF-SynPro pairs. At least 20,000 cells were analyzed for each condition in both histogram and each grid in heat map. Data are recorded by FACS at 24h after cotransfecting.]] | ||
| + | </div> | ||
| + | <div> | ||
| + | <B>Figure 3: the SynTF-SynPro pairs’ Orthogonality. </B>Grids in blue rectangle showed that SynTF-SynPro pairs constructed by using SynZF as DBD with well orthogonality. Grids in pink rectangles replaced our favorite SynTF-SynPro pairs. At least 20,000 cells were analyzed for each condition in both histogram and each grid in heat map. Data are recorded by FACS at 24h after cotransfecting. | ||
| + | |||
| + | </div> | ||
| + | |||
| + | ===Reference=== | ||
| + | [1] L. Morsut et al., Engineering Customized Cell Sensing and Response Behaviors Using Synthetic Notch Receptors. Cell 164, 780--791 (2016). | ||
| + | |||
| + | [2] R. Witzgall, E. O'Leary, A. Leaf, D. Onaldi, J. V. Bonventre, The Krüppel-associated box-A (KRAB-A) domain of zinc finger proteins mediates transcriptional repression. Proceedings of the National Academy of Sciences 91, 4514-4518 (1994). | ||
Latest revision as of 02:28, 1 November 2017
SV40_UAS promoter
A improving of BBa_K511003 MIT_2011
This part (Sv40 UAS ) is a mammalian synthetic promoter (SynPro) based on Sv40 promoter (BBa_K2446052) and 4*GAL4 binding sites from UAS-Gal4 Promoter MammoBlock(BBa_K511003 MIT2011) . We inset the 4*GAL4 binding sites behind the Sv40 promoter. Sv40 promoter is a constitutive expression promoter from the simian vacuolating virus 40. In this way, we can use our mammalian synthetic transcription factors (SynTFs) which is a fusing protein GAL4_DBD_(G4S) linker_NLS_KRAB. The GAL4DBD can bind to the 4*GAL4 binding sites specifically[1] and the KRAB domain can repress the expression of Sv40 UAS promoter [2]. The corresponding SynTF of Sv40-UAS is ZF_GAl4_KRAB (BBa_K2446037).
Figure 1: The design of SynTF-SynPros.
The information of other SynTF-SynPros is showed in the table below.
| SynTFs | SynPros |
|---|---|
| Gal4-KRAB(TF-KRAB-1) BBa_K2446037 | Sv40-UAS(Sv40-UAS) BBa_K2446036 |
| ZF_PIP_KRAB(TF-KRAB-2) BBa_K2446045 | SV40_2/4/8_PIP BBa_K2446033/BBa_K2446034/BBa_K2446035 |
| ZF_21-16KRAB(TF-KRAB-3) BBa_K2446039 | SV40_8_ZF_21-16 BBa_K2446030 |
| ZF_42-10_KRAB(TF-KRAB-4) BBa_K2446040 | SV40_8_ZF_42-10 BBa_K2446025 |
| ZF_43-8_KRAB(TF-KRAB-5) BBa_K2446041 | SV40_2/4/8_ZF_43-8 BBa_K2446026/BBa_K2446027/BBa_K2446028 |
| ZF_54-8_KRAB(TF-KRAB-6) BBa_K2446042 | SV40_8_ZF_54-8 BBa_K2446029 |
| ZFHD1_KRAB(TF-KRAB-7) BBa_K2446043 | SV40_4_ZFHD1 BBa_K2446032 |
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Experiments
SynTF-SynPro Pairs
Figure 1: the testing circuits of GAL4-KRAB& Sv40 UAS pair
To make sure the SynTF-SynPro pairs work in mammalian cells, we use the circuits above to test if the Gal4-KRAB can repress the expression of Sv40-UAS indeed. Gal4-KRAB is linked to the C terminal of EGFP by the link of P2A. And mCherry expressions is controlled by corresponding SynPro (Sv40 UAS). These circuits are both inserted in to the mammalian expression vactor pML2. We transfect pML2-Sv40-UAS into Hela cells and measure the fluorescence intensity of mCherry by flow cytometer to get the basic expression intensity of Sv40-UAS. We also co-transfect the pML2-GAL4-KRAB with pML2-Sv40-UAS into Hela cells at the same time. Then measure the fluorescence intensity of mCherry again to get the expression intensity of Sv40-UAS influenced by GAL4-KRAB. The results of the experiment is showed below. The SynTF GAL4 can silence the expression of the SynPro Sv40-UAS in 25 folds.
Figure 2:The results of GAL4-KRAB&SV40 testing: (A) The red points is the cells before co-transfecting GAL4-KRAB and the blue points is the cells after co-transfecting GAL4-KRAB. It’s easy to see that the red points depart from the diagonal and higher than the blue pints. So the expression of mCherry silenced after the expression of GAL4-KRAB;(B) The red area is the fluorescence intensity of mCherry before co-transfecting GAL4-KRAB and the blue area is the intensity after co-transfecting GAL4-KRAB.(C) The statistical result of all of the SynTFs-SynPros pairs: GAL4-KRAB can silence the expression intensity of Sv40-UAS in 25 folds
SynTF-SynPro Orthogonality
To construct our [http://2017.igem.org/Team:Fudan/Model/GTN| Strip module], more than one SynTF-SynPro pairs would be applied. Thus, the interaction between the pairs would influence or ruin our construction. We did massive orthogonality experiments to avoid that. We observed all of the 5 pairs were actually orthogonal, as you could see the grids on the diagonal were always the darkest. The three DBDs commonly used in previous works were didn’t let us down. However, the expression level of these RE loaded SynPros were relative low compared to SynPro(S)-ZF serials. As the blue rectangle in the lower right corner of the orthogonality may showed the SynPro(S)-ZF has high basic expression with unpaired SynTFs, but could be silenced to the similar fold of commonly used DBDs corresponding SynPros. The SynPro(S)-ZF was likely won’t be target by other unpaired DBD, hence the purple appeared on the bottom rows.
Figure 3: the SynTF-SynPro pairs’ Orthogonality. Grids in blue rectangle showed that SynTF-SynPro pairs constructed by using SynZF as DBD with well orthogonality. Grids in pink rectangles replaced our favorite SynTF-SynPro pairs. At least 20,000 cells were analyzed for each condition in both histogram and each grid in heat map. Data are recorded by FACS at 24h after cotransfecting.
Reference
[1] L. Morsut et al., Engineering Customized Cell Sensing and Response Behaviors Using Synthetic Notch Receptors. Cell 164, 780--791 (2016).
[2] R. Witzgall, E. O'Leary, A. Leaf, D. Onaldi, J. V. Bonventre, The Krüppel-associated box-A (KRAB-A) domain of zinc finger proteins mediates transcriptional repression. Proceedings of the National Academy of Sciences 91, 4514-4518 (1994).