Difference between revisions of "Part:BBa K1989000"
| (20 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| + | <h2>Triplespytag with SUP and His-tag</h2> | ||
| + | <h3>Usage and Biology</h3> | ||
| + | In the last few years, hydrogens made from natural or synthetic polymers have been investigated due to their extensive application in clinical medicine and synthetic biology. Compared to traditional biological material, protein-based multifunctional biological material is low-cost, facile and eco-friendly. However, strategies for assembling 3D molecular networks synthesized only by protein molecular remain underdeveloped. The reason why investigating this technology is still tough is lack of protein-based cross linking agents. Inspired by the self-catalysis of isopeptide bond between Lys and Asp in Streptococcus pyogenes fibronectin-binding protein FbaB, researchers split the catalytic domain and obtained two peptide called SpyTag(the short one) and SpyCatcher(the long one) which are able to form isopeptide bond with the other without any assistant. By fusing SpyTag and SpyCatcher with functional domains respectively, researchers solved the problem tactfully. In order to using SpyTag and SpyCatcher system as scaffold, we fused three SpyTag spaced by (VPGVG)4 with 6xHistag in N-terminal and another functional protein called Super Uranyl-binding Protein(SUP) in C-terminal.This functional protein is obtained from BBa_K1701004 (https://parts.igem.org/Part:BBa_K1701004"). | ||
| + | |||
| + | Another part of this CDS is uranyl-binding domain. Uranium is the key element for nuclear-energy production and is crucial in many other applications. The most stable and relevant uranium ion in aerobic environment is uranyl ion. Super Uranyl-binding Protein(SUP), a completely artificial protein from structure calculating to function modifying is designed to binding uranyl ions specifically. According to the researchers’ result, uranyl-binding affinity and selectivity of SUP is extremely high. The dissociation constant is lower than 10fM which is 1000 folds lower comparing to other common mental ions. | ||
| + | |||
| + | Based on our results, the fused protein His-triple SpyTag-SUP (His-3A-SUP) possess both isopeptide bond forming function and uranyl-binding ability. Thus, using 3A-SUP as a part of hydrogel formation, we could obtain our multifunctional biomaterial. | ||
| + | |||
| + | You can find triplespycatcher here https://parts.igem.org/Part:BBa_K1989001". | ||
| + | |||
| + | <h3>Cultivation, Purification and SDS-PAGE</h3> | ||
| + | |||
| + | <h4>Cultivation</h4> | ||
| + | |||
| + | The part was assembled with T7 promoter and RBS in pET28a plasmid vector. E. coli strain BL21(DE3) harboring the appropriate plasmid was grown at 37 °C in 2xYT medium overnight with suitable concentration of antibiotic. The culture was diluted 100 fold into fresh medium with antibiotic and grown at 37°C to an optical density of 0.6~0.8 at 600 nm, the protein expression was induced with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) and cells were grown overnight at 25°C. | ||
| + | |||
| + | <h4>Purification</h4> | ||
| + | |||
| + | Cells were centrifuged at 8000rpm for 15min at 4°C. Resuspend the cell paste expressing recombinant protein in binding buffer (20 mM Tris-HCl, 0.5 M NaCl, 20 mM imidazole, 1mM β-mercaptoethanol, pH7.4), containing SIGMAFAST™ Protease Inhibitor Cocktail Tablets (SIGMA-ALORICH). Disrupt the cells with sonication for 20 min with suitable power on ice and centrifuge at 18000 rpm for 40 min at 4°C. Remove remaining particles by passing the supernatant through a 0.22 μm filter. | ||
| + | The HisTrap™ column (GE Healthcare, Inc.) was equilibrated with binding buffer. Load the sample and wash the column with binding buffer. | ||
| + | |||
| + | Elute the target protein with a linear gradient starting with binding buffer and ending with the same buffer including 500mM imidazole. The eluted fraction containing the target protein were concentrated by Amicon® Ultra Centrifugal Filters (Merck) with a 10 kDa cutoff, then frozen by liquid nitrogen and stored at -80°C. | ||
| + | |||
| + | <h4>SDS-PAGE</h4> | ||
| + | |||
| + | Protein purification was checked by SDS-PAGE and the resulting protein is quantified by Braford analysis. | ||
| + | |||
| + | <h3>Activity Analysis</h3> | ||
| + | |||
| + | <h4>Gel Formation</h4> | ||
| + | |||
| + | <h5> Basic Exploration</h5> | ||
| + | |||
| + | Exploring the polymerization of our crosslinking network is significant to subsequent application. We must know what our Spy Network is like, and how long they need to complete their reactions, which would offer us a kind of intuition of their crosslinking ability. | ||
| + | |||
| + | To answer the questions shown above, we have analyzed the crosslinking ability of our fundamental monomers, 3A, 3A-SUP, 3A-mSA and 3B. They were firstly diluted to certain concentration, and were made to react under the restriction that the number of A was equal to B. The monomers containing A were conducted to react with 3B at 25℃ and pH=7.3 with the duration of 2 hours. | ||
| + | |||
| + | [[file:Peking_part_cross-1.png|500px]] | ||
| + | |||
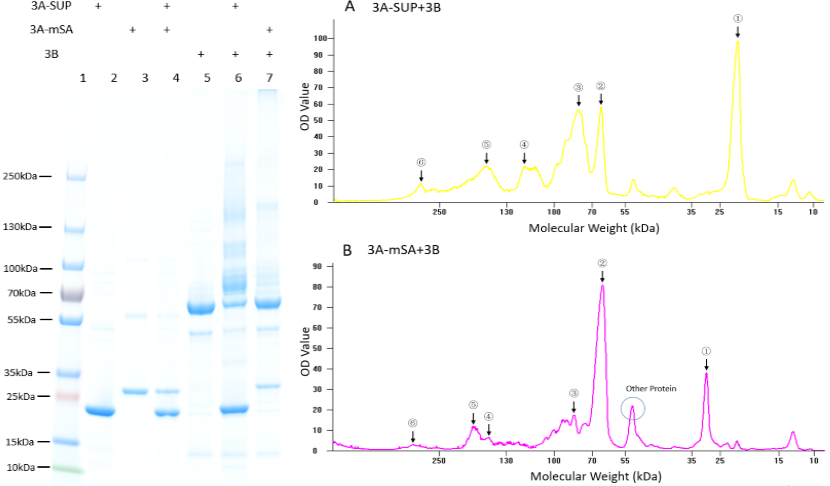
| + | '''Fig. 1. Exploration of the polymerization ability of the 3A-SUP/3A-mSA with 3B. Left: the Coomassie Blue staining gel of basic experiment, which illustrates the basic cross-linking ability of 3A-SUP/3A-mSA and 3B. Lane 1, Thermo® Protein Marker; lane 2, 3A-SUP; lane 3, 3A-mSA; lane 4, 3A-SUP+3A-mSA; lane5, 3B; lane 6, 3A-SUP+3B; lane 7, 3A-mSA+3B. (Molecular Weight: 3A-SUP, 21.4kDa; 3A-mSA, 25.4kDa; 3B, 55.4(62) kDa. Right: A, the OD value of the lane 5 of oligomers produced by the mix of 3A-SUP and 3B. Peaks illustrate the monomers and the possible products, ①, 3A-SUP (21.4kDa); ②, 3B (62kDa); ③, 1x 3A-SUP+1x 3B (83.4kDa); ④, 3x 3A-SUP+1x 3B (126.2kDa); ⑤, 2x 3A-SUP+2x 3B (166.8kDa); ⑥, 4x 3A-SUP+4x 3B (333.6kDa). B, the OD value of the lane 6 of oligomers produced by the mix of 3A-mSA and 3B. Peaks illustrate the monomers and the possible products, ①, 3A-mSA (25.4kDa); ②, 3B (62kDa); ③, 1x 3A-mSA+1x 3B (87.4kDa); ④, 1x 3A-mSA+2x 3B (149.4kDa); ⑤, 2x 3A-mSA+2x 3B (174.4kDa); ⑥, 3x 3A-mSA+4x 3B (324.2kDa). (The software lane 1D was used to draw the graph.) (“3A-SUP” stands for “Triple SpyTag-SUP”, “3A-mSA” for “Triple SpyTag-mSA”, and “3B” is the abbreviation of “Triple SpyCatcher”)''' | ||
| + | |||
| + | We found that some new bands appeared above the band of 3B when it was mixed with monomers containing A, which demonstrated that our idea of forming functional hydrogel was executable. The products were mainly oligomers (Fig. 2 A-F, Table 1), for it is easy to form loops, which hindered the linkage between different monomers at such low concentration. Interestingly, with the restriction that A is equal to B in number, and the content of 3B was constant initially, the crosslinking ability at low concentration of these monomers were different with each other by comparing the surplus content of 3B. What’s more, it was surprising that the position of 3B band (~62kDa) was not accord with its theoretical weight (55.4kDa). Further experiments will be done to understand the differences. | ||
| + | |||
| + | <h5>Gradient Experiment</h5> | ||
| + | |||
| + | After exploring the basic character, i.e., the ability to crosslink, of our reactants, we would like to further explore the advantageous conditions for our polymerization, such as pH gradient, temperature gradient, and concentration gradient of monomers. | ||
| + | |||
| + | [[file:Peking_part_cross-D.png|500px]] | ||
| + | |||
| + | '''Fig. 2. Design the gradient experiment of pH, temperature and concentration''' | ||
| + | |||
| + | First experiment is concerned with pH gradient. Given that our Spy Network in practical application would face water environment with pH nearly neutral, and our proteins have their own optimum pH of functioning, we decide to make pH gradient of 6.3/7.3/8.3. Typical monomers 3A-SUP and 3B would firstly dissolve in TBS at certain pH values, then they were put to react at mentioned pH gradient. The reaction would be conducted at 25℃, with duration of 2 hours. Samples would be extracted every 30min to make clear the variation tendency of monomers. After the reaction, SDS-PAGE would be conducted and the gels would be scanned and analyzed by the software Lane 1D. Thus the relationship between time and the content of 3B and 3A would be found out. Three parallel groups were set to realize quantitative analysis. | ||
| + | |||
| + | [[file:Peking_part_cross-2.png|500px]] | ||
| + | |||
| + | '''Fig. 3. pH Gradient Experiment A: the reactive extent for 3B at different pH. Experiments were repeated three times and error bars were added. B: The mass distribution of oligomers at different time at pH7.3 (70kDa-450kDa).''' | ||
| + | |||
| + | From the results of analyzing SDS-PAGE gels showing above, we clearly observed the changing law of 3B and the mass distribution of polymer homologs. Appropriately lower pH would lead to faster consumption of monomers, while our time scale should be smaller in order to show the condensation polymerization nature. | ||
| + | |||
| + | Second is concerned with temperature. According to the common environmental temperature, three typical values, 16℃, 25℃, 37℃ were introduced in our temperature experiment. Similar to pH Gradient Experiment, typical monomers 3A-SUP and 3B would firstly dissolve in TBS at pH=7.3, then they were put to react at mentioned temperature gradient. The reaction would be conducted at pH=7.3, with the duration of 2 hours. The subsequent steps were same to that of pH Gradient Experiment. | ||
| + | |||
| + | [[file:Peking_part_cross-3.png|500px]] | ||
| + | |||
| + | '''Fig. 4. Temperature Gradient Experiment A: the reactive extent for 3B at different temperature. Experiments were repeated three times and error bars were added. B: The mass distribution of oligomers at different time at 16℃ (70kDa-400kDa).''' | ||
| + | |||
| + | The analysis of SDS-PAGE gel clearly showed the variation tendency of the surplus content of 3B as a function of time, and of the mass distribution of homologs. According to the result, we could conclude that higher temperature would lead to the faster consumption of monomer, and at designated time scale we didn’t see any change in the change of weight distribution of polymer homologs. We supposed that it should be easier to form loops (intramolecular reactions) at such low concentration, and the time scale is too large to show the variation. Though the rate of polymerization should be described by the consumption rate of functional groups, we decide to use the consumption rate of monomers to simply describe the tendency. | ||
| + | |||
| + | Third is concerned with concentration. At low concentration would cross-linkage not polymerize, but oligomerize (The difference between these two reactions can be described by crosslinking density, the former is large, while the latter is rather small.), according to the basic knowledge of Polymer Science. From this point of view, if we hope to promote the mass, strength and contact area of polymers, we have to mix our monomers at high concentration. Thus, understand the relationship between the concentration and the variation of mass distribution of polymers is quite essential. | ||
| + | |||
| + | [[file:Peking_part_conc-grad.png|500px]] | ||
| + | |||
| + | '''Fig. 5. The mass distribution of oligomers of concentration gradient experiment at different concentration Left: the Coomassie Blue staining gel of concentration gradient experiment, which clearly showed the change of mass distribution according to the change of concentration. Lane 1, Thermo® Protein Marker; lane 2, 3A-SUP; lane 3, 3A-mSA; lane 4, 3A-SUP+3A-mSA; lane5, 3B; (1mg/mL) lane 6, 3A-SUP+3B; lane 7, 3A-mSA+3B; lane 8, 3A-SUP+3A-mSA+3B; (5mg/mL) lane 9, 3A-SUP+3B; lane 10, 3A-mSA+3B; lane 11, 3A-SUP+3A-mSA+3B; (10mg/mL) lane 12, 3A-SUP+3B; lane 13, 3A-SUP+3A-mSA(5mg/mL)+3B. (Molecular Weight: 3A-SUP, 21.4kDa; 3A-mSA, 25.4kDa; 3B, 55.4(62)kDa. “3A-SUP” stands for “Triple SpyTag-SUP”, “3A-mSA” for “Triple SpyTag-mSA”, and “3B” is the abbreviation of “Triple SpyCatcher”). 5mg/mL and 10mg/mL samples were diluted to 1mg/mL after the reaction (with the duration of 2 hours). The mass of samples in lanes was equal to each other. Right: the OD value of the lanes 2, 5, 6, 9, 12 of the oligomers produced by the mix of 3A-SUP and 3B. Peaks illustrate the monomers and the possible products, ①, 3A-SUP (21.4kDa); ②, 3B (62kDa); ③, 1x 3A-SUP+1x 3B (83.4kDa); ④, 3x 3A-SUP+1x 3B (126.2kDa); ⑤, 2x 3A-SUP+2x 3B (166.8kDa); ⑥, 4x 3A-SUP+4x 3B (333.6kDa); ⑦, average mass of polymers larger than 750kDa (~6x 3A-sup+10x 3B or 9x 3A-SUP+9x 3B). (The software lane 1D was used to draw the graph.)''' | ||
| + | |||
| + | The analysis of SDS-PAGE gel showed that more hyper-branched products with heavier molecular weight appeared with the increase of concentration, which, could also lead to the prediction that the average weight would be larger if higher concentration of monomers were mixed together. | ||
| + | |||
| + | <h4>Uranyl-binding affinity and selectivity</h4> | ||
| + | |||
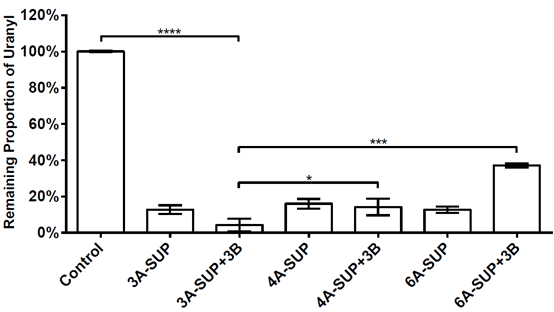
| + | We tested the adsorption capacity of 3A-SUP, 4A-SUP, 6A-SUP and 3A-SUP+3B, 4A-SUP+3B, 6A-SUP+3B in TBS buffer (pH=7.02) against 10μM uranyl. We wanted to do some comparisons and found the best candidate. Cross-linked 3A-SUP+3B can sequester 95.77% of the total uranyl in TBS buffer (pH=7.0, 10μM), showing the best adsorption capacity. Other proteins also can sequester at least 60% of the total uranyl. The standard deviation were calculated from triplicate experiments. | ||
| + | |||
| + | [[file:Peking_part_sup-1-c.png|500px]] | ||
| + | |||
| + | '''Figure. 6. Adsorption capacity of 3A-SUP,4A-SUP,6A-SUP and 3A-SUP+3B,4A-SUP+3B,6A-SUP+3B''' | ||
| + | |||
| + | We then test different protein-uranyl ratio in TBS buffer against 10μM uranyl. The use of one and ten equivalents of protein against one equivalent of uranyl (10μM) in TBS buffer can sequester 89.79% and 92.70% of the total uranyl respectively. When protein-uranyl ratio increased, the adsorption rate also increased, but not significantly. The standard deviation were calculated from triplicate experiments. | ||
| + | |||
| + | [[file:Peking_part_sup-2.png|500px]] | ||
| + | |||
| + | '''Figure. 7. Adsorption capacity of 3A-SUP+3B with different protein-uranyl ratio in TBS buffer''' | ||
| + | |||
| + | To test whether our protein can work in real water condition, we use lake and synthetic sea water as solutions. 3A-SUP+3B can sequester 89.79% and 26.88% and 89.93% of the total uranyl respectively in TBS buffer, lake water and synthetic sea water. The standard deviation were calculated from triplicate experiments. We find that when proteins were employed in lake water, the adsorption decreased, the reason might be that other creatures in lake water interferes the proteins’ function. | ||
| + | |||
| + | [[file:Peking_part_sup-3.png|500px]] | ||
| + | |||
| + | '''Figure. 8. Adsorption capacity of 3A-SUP+3B in different water conditions''' | ||
| + | |||
| + | Furthermore, we decreased the uranyl concentration to 13nM and increased the equivalent of the proteins to test the sensitivity of 3A-SUP+3B in synthetic TBS buffer and sea water. To test the adsorption sensitivity, thee use of 6000 equivalent of protein against one equivalent of uranyl (13nM) in synthetic sea water and TBS buffer can remove 33.11% and 47.48% respectively. The concentration was determined by ICP-MS. | ||
| + | |||
| + | [[file:Peking_part_sup-4.png|500px]] | ||
| + | |||
| + | '''Figure 9. Adsorption capacity of 3A-SUP+3B against 13 nM uranyl in different water conditions: 6000 equivalent of 3A-SUP+3B against one equivalent of uranyl (13nM) in TBS buffer and synthetic sea water.''' | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | ==References== | ||
| + | 1. Zhou, Lu. et al. Nature chemistry 6, 236-241 (2014). | ||
| + | |||
| + | 2. Sun, F. et al. Proc. Natl Acad. Sci. USA 111, 11269-11274 (2014). | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <span class='h3bb'>Sequence and Features</span> | ||
| + | <partinfo>BBa_K1989000 SequenceAndFeatures</partinfo> | ||
| + | |||
| + | |||
| + | ===Functional Parameters=== | ||
| + | <partinfo>BBa_K1989000 parameters</partinfo> | ||
Latest revision as of 10:25, 16 October 2016
Triplespytag with SUP and His-tag
Usage and Biology
In the last few years, hydrogens made from natural or synthetic polymers have been investigated due to their extensive application in clinical medicine and synthetic biology. Compared to traditional biological material, protein-based multifunctional biological material is low-cost, facile and eco-friendly. However, strategies for assembling 3D molecular networks synthesized only by protein molecular remain underdeveloped. The reason why investigating this technology is still tough is lack of protein-based cross linking agents. Inspired by the self-catalysis of isopeptide bond between Lys and Asp in Streptococcus pyogenes fibronectin-binding protein FbaB, researchers split the catalytic domain and obtained two peptide called SpyTag(the short one) and SpyCatcher(the long one) which are able to form isopeptide bond with the other without any assistant. By fusing SpyTag and SpyCatcher with functional domains respectively, researchers solved the problem tactfully. In order to using SpyTag and SpyCatcher system as scaffold, we fused three SpyTag spaced by (VPGVG)4 with 6xHistag in N-terminal and another functional protein called Super Uranyl-binding Protein(SUP) in C-terminal.This functional protein is obtained from BBa_K1701004 (https://parts.igem.org/Part:BBa_K1701004").
Another part of this CDS is uranyl-binding domain. Uranium is the key element for nuclear-energy production and is crucial in many other applications. The most stable and relevant uranium ion in aerobic environment is uranyl ion. Super Uranyl-binding Protein(SUP), a completely artificial protein from structure calculating to function modifying is designed to binding uranyl ions specifically. According to the researchers’ result, uranyl-binding affinity and selectivity of SUP is extremely high. The dissociation constant is lower than 10fM which is 1000 folds lower comparing to other common mental ions.
Based on our results, the fused protein His-triple SpyTag-SUP (His-3A-SUP) possess both isopeptide bond forming function and uranyl-binding ability. Thus, using 3A-SUP as a part of hydrogel formation, we could obtain our multifunctional biomaterial.
You can find triplespycatcher here https://parts.igem.org/Part:BBa_K1989001".
Cultivation, Purification and SDS-PAGE
Cultivation
The part was assembled with T7 promoter and RBS in pET28a plasmid vector. E. coli strain BL21(DE3) harboring the appropriate plasmid was grown at 37 °C in 2xYT medium overnight with suitable concentration of antibiotic. The culture was diluted 100 fold into fresh medium with antibiotic and grown at 37°C to an optical density of 0.6~0.8 at 600 nm, the protein expression was induced with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) and cells were grown overnight at 25°C.
Purification
Cells were centrifuged at 8000rpm for 15min at 4°C. Resuspend the cell paste expressing recombinant protein in binding buffer (20 mM Tris-HCl, 0.5 M NaCl, 20 mM imidazole, 1mM β-mercaptoethanol, pH7.4), containing SIGMAFAST™ Protease Inhibitor Cocktail Tablets (SIGMA-ALORICH). Disrupt the cells with sonication for 20 min with suitable power on ice and centrifuge at 18000 rpm for 40 min at 4°C. Remove remaining particles by passing the supernatant through a 0.22 μm filter. The HisTrap™ column (GE Healthcare, Inc.) was equilibrated with binding buffer. Load the sample and wash the column with binding buffer.
Elute the target protein with a linear gradient starting with binding buffer and ending with the same buffer including 500mM imidazole. The eluted fraction containing the target protein were concentrated by Amicon® Ultra Centrifugal Filters (Merck) with a 10 kDa cutoff, then frozen by liquid nitrogen and stored at -80°C.
SDS-PAGE
Protein purification was checked by SDS-PAGE and the resulting protein is quantified by Braford analysis.
Activity Analysis
Gel Formation
Basic Exploration
Exploring the polymerization of our crosslinking network is significant to subsequent application. We must know what our Spy Network is like, and how long they need to complete their reactions, which would offer us a kind of intuition of their crosslinking ability.
To answer the questions shown above, we have analyzed the crosslinking ability of our fundamental monomers, 3A, 3A-SUP, 3A-mSA and 3B. They were firstly diluted to certain concentration, and were made to react under the restriction that the number of A was equal to B. The monomers containing A were conducted to react with 3B at 25℃ and pH=7.3 with the duration of 2 hours.
Fig. 1. Exploration of the polymerization ability of the 3A-SUP/3A-mSA with 3B. Left: the Coomassie Blue staining gel of basic experiment, which illustrates the basic cross-linking ability of 3A-SUP/3A-mSA and 3B. Lane 1, Thermo® Protein Marker; lane 2, 3A-SUP; lane 3, 3A-mSA; lane 4, 3A-SUP+3A-mSA; lane5, 3B; lane 6, 3A-SUP+3B; lane 7, 3A-mSA+3B. (Molecular Weight: 3A-SUP, 21.4kDa; 3A-mSA, 25.4kDa; 3B, 55.4(62) kDa. Right: A, the OD value of the lane 5 of oligomers produced by the mix of 3A-SUP and 3B. Peaks illustrate the monomers and the possible products, ①, 3A-SUP (21.4kDa); ②, 3B (62kDa); ③, 1x 3A-SUP+1x 3B (83.4kDa); ④, 3x 3A-SUP+1x 3B (126.2kDa); ⑤, 2x 3A-SUP+2x 3B (166.8kDa); ⑥, 4x 3A-SUP+4x 3B (333.6kDa). B, the OD value of the lane 6 of oligomers produced by the mix of 3A-mSA and 3B. Peaks illustrate the monomers and the possible products, ①, 3A-mSA (25.4kDa); ②, 3B (62kDa); ③, 1x 3A-mSA+1x 3B (87.4kDa); ④, 1x 3A-mSA+2x 3B (149.4kDa); ⑤, 2x 3A-mSA+2x 3B (174.4kDa); ⑥, 3x 3A-mSA+4x 3B (324.2kDa). (The software lane 1D was used to draw the graph.) (“3A-SUP” stands for “Triple SpyTag-SUP”, “3A-mSA” for “Triple SpyTag-mSA”, and “3B” is the abbreviation of “Triple SpyCatcher”)
We found that some new bands appeared above the band of 3B when it was mixed with monomers containing A, which demonstrated that our idea of forming functional hydrogel was executable. The products were mainly oligomers (Fig. 2 A-F, Table 1), for it is easy to form loops, which hindered the linkage between different monomers at such low concentration. Interestingly, with the restriction that A is equal to B in number, and the content of 3B was constant initially, the crosslinking ability at low concentration of these monomers were different with each other by comparing the surplus content of 3B. What’s more, it was surprising that the position of 3B band (~62kDa) was not accord with its theoretical weight (55.4kDa). Further experiments will be done to understand the differences.
Gradient Experiment
After exploring the basic character, i.e., the ability to crosslink, of our reactants, we would like to further explore the advantageous conditions for our polymerization, such as pH gradient, temperature gradient, and concentration gradient of monomers.
Fig. 2. Design the gradient experiment of pH, temperature and concentration
First experiment is concerned with pH gradient. Given that our Spy Network in practical application would face water environment with pH nearly neutral, and our proteins have their own optimum pH of functioning, we decide to make pH gradient of 6.3/7.3/8.3. Typical monomers 3A-SUP and 3B would firstly dissolve in TBS at certain pH values, then they were put to react at mentioned pH gradient. The reaction would be conducted at 25℃, with duration of 2 hours. Samples would be extracted every 30min to make clear the variation tendency of monomers. After the reaction, SDS-PAGE would be conducted and the gels would be scanned and analyzed by the software Lane 1D. Thus the relationship between time and the content of 3B and 3A would be found out. Three parallel groups were set to realize quantitative analysis.
Fig. 3. pH Gradient Experiment A: the reactive extent for 3B at different pH. Experiments were repeated three times and error bars were added. B: The mass distribution of oligomers at different time at pH7.3 (70kDa-450kDa).
From the results of analyzing SDS-PAGE gels showing above, we clearly observed the changing law of 3B and the mass distribution of polymer homologs. Appropriately lower pH would lead to faster consumption of monomers, while our time scale should be smaller in order to show the condensation polymerization nature.
Second is concerned with temperature. According to the common environmental temperature, three typical values, 16℃, 25℃, 37℃ were introduced in our temperature experiment. Similar to pH Gradient Experiment, typical monomers 3A-SUP and 3B would firstly dissolve in TBS at pH=7.3, then they were put to react at mentioned temperature gradient. The reaction would be conducted at pH=7.3, with the duration of 2 hours. The subsequent steps were same to that of pH Gradient Experiment.
Fig. 4. Temperature Gradient Experiment A: the reactive extent for 3B at different temperature. Experiments were repeated three times and error bars were added. B: The mass distribution of oligomers at different time at 16℃ (70kDa-400kDa).
The analysis of SDS-PAGE gel clearly showed the variation tendency of the surplus content of 3B as a function of time, and of the mass distribution of homologs. According to the result, we could conclude that higher temperature would lead to the faster consumption of monomer, and at designated time scale we didn’t see any change in the change of weight distribution of polymer homologs. We supposed that it should be easier to form loops (intramolecular reactions) at such low concentration, and the time scale is too large to show the variation. Though the rate of polymerization should be described by the consumption rate of functional groups, we decide to use the consumption rate of monomers to simply describe the tendency.
Third is concerned with concentration. At low concentration would cross-linkage not polymerize, but oligomerize (The difference between these two reactions can be described by crosslinking density, the former is large, while the latter is rather small.), according to the basic knowledge of Polymer Science. From this point of view, if we hope to promote the mass, strength and contact area of polymers, we have to mix our monomers at high concentration. Thus, understand the relationship between the concentration and the variation of mass distribution of polymers is quite essential.
Fig. 5. The mass distribution of oligomers of concentration gradient experiment at different concentration Left: the Coomassie Blue staining gel of concentration gradient experiment, which clearly showed the change of mass distribution according to the change of concentration. Lane 1, Thermo® Protein Marker; lane 2, 3A-SUP; lane 3, 3A-mSA; lane 4, 3A-SUP+3A-mSA; lane5, 3B; (1mg/mL) lane 6, 3A-SUP+3B; lane 7, 3A-mSA+3B; lane 8, 3A-SUP+3A-mSA+3B; (5mg/mL) lane 9, 3A-SUP+3B; lane 10, 3A-mSA+3B; lane 11, 3A-SUP+3A-mSA+3B; (10mg/mL) lane 12, 3A-SUP+3B; lane 13, 3A-SUP+3A-mSA(5mg/mL)+3B. (Molecular Weight: 3A-SUP, 21.4kDa; 3A-mSA, 25.4kDa; 3B, 55.4(62)kDa. “3A-SUP” stands for “Triple SpyTag-SUP”, “3A-mSA” for “Triple SpyTag-mSA”, and “3B” is the abbreviation of “Triple SpyCatcher”). 5mg/mL and 10mg/mL samples were diluted to 1mg/mL after the reaction (with the duration of 2 hours). The mass of samples in lanes was equal to each other. Right: the OD value of the lanes 2, 5, 6, 9, 12 of the oligomers produced by the mix of 3A-SUP and 3B. Peaks illustrate the monomers and the possible products, ①, 3A-SUP (21.4kDa); ②, 3B (62kDa); ③, 1x 3A-SUP+1x 3B (83.4kDa); ④, 3x 3A-SUP+1x 3B (126.2kDa); ⑤, 2x 3A-SUP+2x 3B (166.8kDa); ⑥, 4x 3A-SUP+4x 3B (333.6kDa); ⑦, average mass of polymers larger than 750kDa (~6x 3A-sup+10x 3B or 9x 3A-SUP+9x 3B). (The software lane 1D was used to draw the graph.)
The analysis of SDS-PAGE gel showed that more hyper-branched products with heavier molecular weight appeared with the increase of concentration, which, could also lead to the prediction that the average weight would be larger if higher concentration of monomers were mixed together.
Uranyl-binding affinity and selectivity
We tested the adsorption capacity of 3A-SUP, 4A-SUP, 6A-SUP and 3A-SUP+3B, 4A-SUP+3B, 6A-SUP+3B in TBS buffer (pH=7.02) against 10μM uranyl. We wanted to do some comparisons and found the best candidate. Cross-linked 3A-SUP+3B can sequester 95.77% of the total uranyl in TBS buffer (pH=7.0, 10μM), showing the best adsorption capacity. Other proteins also can sequester at least 60% of the total uranyl. The standard deviation were calculated from triplicate experiments.
Figure. 6. Adsorption capacity of 3A-SUP,4A-SUP,6A-SUP and 3A-SUP+3B,4A-SUP+3B,6A-SUP+3B
We then test different protein-uranyl ratio in TBS buffer against 10μM uranyl. The use of one and ten equivalents of protein against one equivalent of uranyl (10μM) in TBS buffer can sequester 89.79% and 92.70% of the total uranyl respectively. When protein-uranyl ratio increased, the adsorption rate also increased, but not significantly. The standard deviation were calculated from triplicate experiments.
Figure. 7. Adsorption capacity of 3A-SUP+3B with different protein-uranyl ratio in TBS buffer
To test whether our protein can work in real water condition, we use lake and synthetic sea water as solutions. 3A-SUP+3B can sequester 89.79% and 26.88% and 89.93% of the total uranyl respectively in TBS buffer, lake water and synthetic sea water. The standard deviation were calculated from triplicate experiments. We find that when proteins were employed in lake water, the adsorption decreased, the reason might be that other creatures in lake water interferes the proteins’ function.
Figure. 8. Adsorption capacity of 3A-SUP+3B in different water conditions
Furthermore, we decreased the uranyl concentration to 13nM and increased the equivalent of the proteins to test the sensitivity of 3A-SUP+3B in synthetic TBS buffer and sea water. To test the adsorption sensitivity, thee use of 6000 equivalent of protein against one equivalent of uranyl (13nM) in synthetic sea water and TBS buffer can remove 33.11% and 47.48% respectively. The concentration was determined by ICP-MS.
Figure 9. Adsorption capacity of 3A-SUP+3B against 13 nM uranyl in different water conditions: 6000 equivalent of 3A-SUP+3B against one equivalent of uranyl (13nM) in TBS buffer and synthetic sea water.
References
1. Zhou, Lu. et al. Nature chemistry 6, 236-241 (2014).
2. Sun, F. et al. Proc. Natl Acad. Sci. USA 111, 11269-11274 (2014).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]