Difference between revisions of "Part:BBa K1321100"
| (2 intermediate revisions by 2 users not shown) | |||
| Line 6: | Line 6: | ||
This construct is part of a library of fusions with cellulose binding domains which we designed to bind to cellulose and enable capture of heavy metals ([http://2014.igem.org/Team:Imperial/Functionalisation project page]). Other fusion parts with this metal binding protein can be seen in the table below: [[File:IC14-PC-part-table.PNG]] | This construct is part of a library of fusions with cellulose binding domains which we designed to bind to cellulose and enable capture of heavy metals ([http://2014.igem.org/Team:Imperial/Functionalisation project page]). Other fusion parts with this metal binding protein can be seen in the table below: [[File:IC14-PC-part-table.PNG]] | ||
| − | Note that the | + | Note that the stop codon plus 6 bp at the end of the sequence are included the RFC25 suffix which is not shown. The prefix to this part is RFC10 format. |
| + | |||
| + | For reference the cellulose binding domain binding capability to bacterial cellulose was measured relative to other cellulose binding domains when fused to sfGFP, the data for which can be seen here ([https://parts.igem.org/Part:BBa_K1321348 here]) - K1321348. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | As part of our project, we needed to assay the metal binding capability of our Phytochelatins fused to CBDs (cellulose binding domains). The parts used for this assay are | ||
| + | Phytochelatin+CBDcex, Phytochelatin+dCBD, CBDcipA+Phytochelatin, Phytochelatin alone and sfGFP+dCBD wash (only one wash). | ||
| + | |||
| + | Phytochelatin fused to our CBDs were bound onto cellulose that were dried in the bottom of 96-well plates and tested against 3 different metals (nickel, copper, zinc). | ||
| + | First, the fusion protein cell lysate was incubated overnight in the cellulose wells. Following this, the metal salt solutions are added in excess into the wells. | ||
| + | Finally, an EDTA step removes the bound metal ions into solution, and the metal concentration in solution is quantified by mass spectrometer. Multiple washes with PBS and water were done between each binding step, ensuring that the metal ions that are measured were released from the phytochelatin. | ||
| + | |||
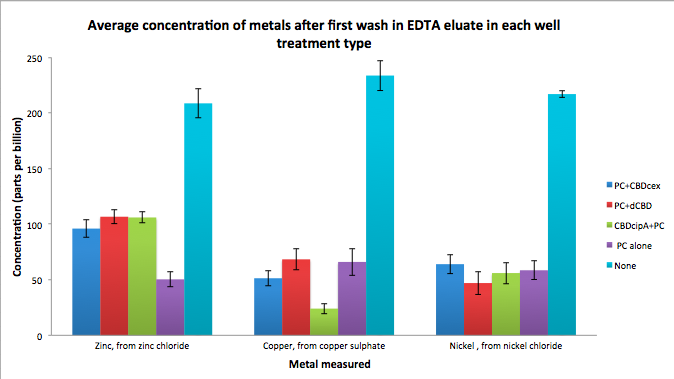
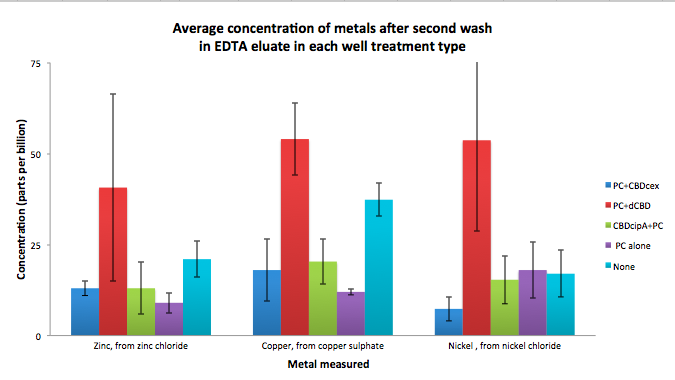
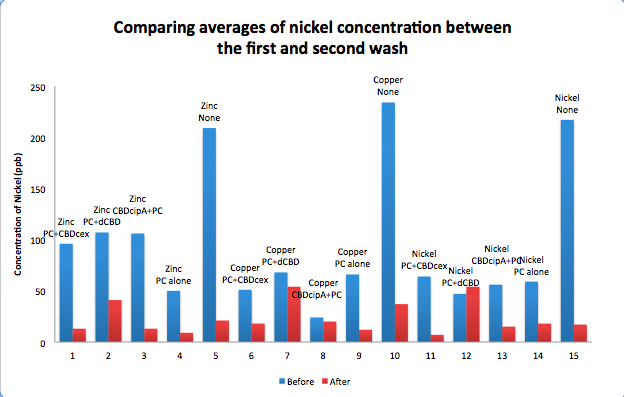
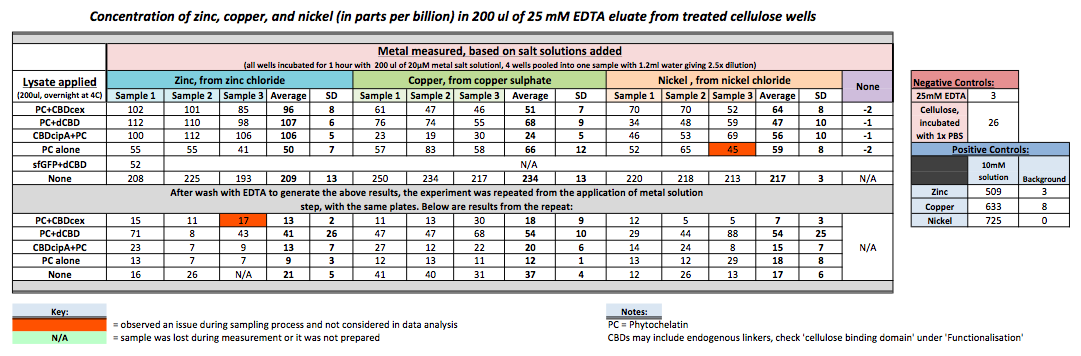
| + | To evaluate if these CBD fusions were reusable, we re-applied metal ion solutions onto the same wells with the CBD fusions adhered. We washed the wells as before, then eluted with EDTA in the same manner. The results from the each elutions are shown below, with the final graph comparing between the first and second wash. We found along the way our bacterial cellulose alone had some metal chelating properties, as was also confirmed by our filtering set up with the dCBD-phytochelatin, but the second elution seems to show less background noise as the EDTA disrupts cellulose's natural binding to the metal ions. The full table of results are shown below as well. The full assay protocol can be found as ‘Metal binding assay protocol’ at this link: | ||
| + | http://2014.igem.org/Team:Imperial/Protocols | ||
| + | |||
| + | |||
| + | To read more about this assay and explanation of these results, please see: | ||
| + | http://2014.igem.org/Team:Imperial/Water_Filtration | ||
| + | |||
| + | [[File:IC14_first_wash_CBD_metal_conc.png|700px|left|]] | ||
| + | |||
| + | [[File:IC14_second_wash_CBD_metal_conc.png|700px|left|]] | ||
| + | |||
| + | [[File:IC14_Comparison_Nickel_conc_washes_CBDs.png|700px|left|]] | ||
| + | |||
| + | [[File:IC-2014_metalbindingtable.jpg|900px|left|]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
Latest revision as of 04:36, 2 November 2014
Phytochelatin (PC) EC20 fused to linker-dCBD with T7 promoter
A T7 promoter expression construct of synthetic phytochelatin EC20 (metal binding peptide) fused N-terminally to dCBD (a cellulose-binding domain), which contains an endogenous N-terminal linker sequence.
This construct is part of a library of fusions with cellulose binding domains which we designed to bind to cellulose and enable capture of heavy metals ([http://2014.igem.org/Team:Imperial/Functionalisation project page]). Other fusion parts with this metal binding protein can be seen in the table below: 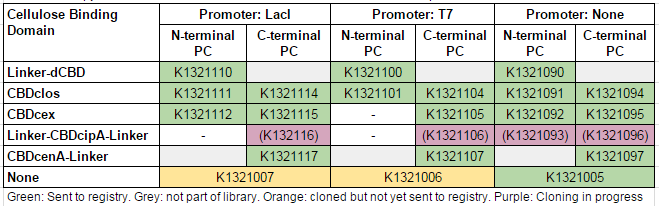
Note that the stop codon plus 6 bp at the end of the sequence are included the RFC25 suffix which is not shown. The prefix to this part is RFC10 format.
For reference the cellulose binding domain binding capability to bacterial cellulose was measured relative to other cellulose binding domains when fused to sfGFP, the data for which can be seen here (here) - K1321348.
As part of our project, we needed to assay the metal binding capability of our Phytochelatins fused to CBDs (cellulose binding domains). The parts used for this assay are
Phytochelatin+CBDcex, Phytochelatin+dCBD, CBDcipA+Phytochelatin, Phytochelatin alone and sfGFP+dCBD wash (only one wash).
Phytochelatin fused to our CBDs were bound onto cellulose that were dried in the bottom of 96-well plates and tested against 3 different metals (nickel, copper, zinc). First, the fusion protein cell lysate was incubated overnight in the cellulose wells. Following this, the metal salt solutions are added in excess into the wells. Finally, an EDTA step removes the bound metal ions into solution, and the metal concentration in solution is quantified by mass spectrometer. Multiple washes with PBS and water were done between each binding step, ensuring that the metal ions that are measured were released from the phytochelatin.
To evaluate if these CBD fusions were reusable, we re-applied metal ion solutions onto the same wells with the CBD fusions adhered. We washed the wells as before, then eluted with EDTA in the same manner. The results from the each elutions are shown below, with the final graph comparing between the first and second wash. We found along the way our bacterial cellulose alone had some metal chelating properties, as was also confirmed by our filtering set up with the dCBD-phytochelatin, but the second elution seems to show less background noise as the EDTA disrupts cellulose's natural binding to the metal ions. The full table of results are shown below as well. The full assay protocol can be found as ‘Metal binding assay protocol’ at this link: http://2014.igem.org/Team:Imperial/Protocols
To read more about this assay and explanation of these results, please see:
http://2014.igem.org/Team:Imperial/Water_Filtration
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 53
- 1000COMPATIBLE WITH RFC[1000]