Difference between revisions of "Part:BBa K1172915"
| (2 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
<partinfo>BBa_K1172915 short</partinfo> | <partinfo>BBa_K1172915 short</partinfo> | ||
| − | Biosafety-System TetOR alive | + | <p align="justify"> |
| + | The Biosafety-System TetOR alive <bbpart>BBa_K1172915</bbpart> is an improvement of the BioBrick <bbpart>BBa_K914014</bbpart> by replacing the first promoter into the rhamnose promoter P<sub>''Rha''</sub>, integration of the alanine racemase <bbpart>BBa_K1172901</bbpart> and utilization of the repressor TetR to regulate the transcription of the Barnase behind the ''tetO'' promoter. Because this system is known for a tight repression and a fast activation, it is expected that bacteria containing this Biosafety-System are dead or alive... | ||
| + | </p> | ||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
| Line 11: | Line 13: | ||
<partinfo>BBa_K1172915 SequenceAndFeatures</partinfo> | <partinfo>BBa_K1172915 SequenceAndFeatures</partinfo> | ||
| − | [[File: | + | ==='''Biosafety system TetOR alive'''=== |
| + | <br> | ||
| + | <p align="justify"> | ||
| + | Combining the genes described above with the Biosafety-Strain K-12 ∆''alr'' ∆''dadX'' results in a powerful device, allowing us to control the bacterial cell division. The control of the bacterial growth is possible either active or passive. Active by inducing the ''tetO'' operator with tetracycline and passive by the induction of L-rhamnose. The passive control makes it possible to control the bacterial cell division in a defined closed environment, like the MFC, by continuously adding L-rhamnose to the medium. As shown in Figure 1 below, this leads to an expression of the essential alanine racemase (''alr'') and the TetR repressor, so that the expression of the RNase Ba is repressed. </p><br> | ||
| + | [[File:IGEM Bielefeld 2013 Biosafety System M 2.png|600px|thumb|center|'''Figure 1:'''Biosafety-System ''TetOR alive'' in the presence of L-rhamnose. The essential alanine racemase (Alr) and the repressor TetR are expressed, resulting in a repression of the expression of the RNAse Ba. Consequently the bacteria show normal growth behavior.]] | ||
| + | <br> | ||
| + | <p align="justify"> | ||
| + | In the event that bacteria exit the defined environment of the MFC or L-rhamnose is not added to the medium any more, both the expression of the alanine racemase (Alr) and the TetR repressor decrease, so that the expression of the toxic RNase Ba (Barnase) begins. The cleavage of the intracellular RNA by the Barnase and the lack of synthesized D-alanine, caused by the repressed alanine racemase inhibit the cell division. Through this it can be secured that the bacteria can only grow in the defined area or the device of choice respectively. </p><br> | ||
| − | [[File: | + | [[File:IGEM Bielefeld 2013 Biosafety System M ohne Rhamnose+ 2.png|600px|thumb|center|'''Figure 2:'''Active Biosafety-System ''TetOR alive'' outside of a defined environment lacking L-rhamnose. Both the expression of the alanine racemase (Alr) and TetR repressor are reduced and ideally completely shut down. In contrast, the expression of the RNase Ba (Barnase) is turned on, leading to cell death by RNA cleavage.]] |
| + | <br> | ||
| + | ==Results== | ||
| + | <br> | ||
| + | ==='''Characterization of the tetracycline ''tetO'' operator'''=== | ||
| + | <p align="justify"> | ||
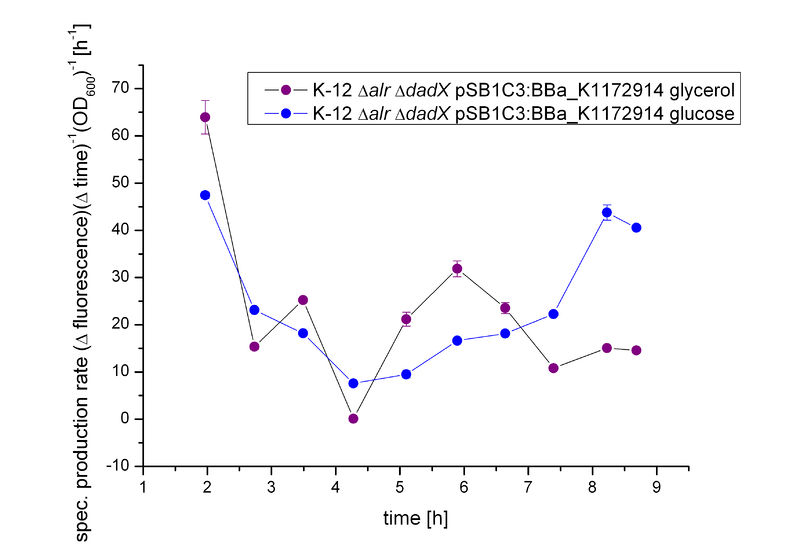
| + | First, the ''tetO'' operator was characterized to get a first impression of its basal transcription rate. Therefore, the bacterial growth was investigated using the unrepressed ''tetO'' operator on different carbon source using M9 minimal medium with either glucose or glycerol. The transcription rate was identified by fluorescence measurement of GFP <bbpart>BBa_E0040</bbpart> behind the ''tetO'' operator <bbpart>BBa_R0040</bbpart> using the BioBrick <bbpart>BBa_K1172914</bbpart>.<br> | ||
| + | As shown in Figure 9 below, the bacteria grew slightly better on glucose then on glycerol. This is due to glucose being the better energy source of these two, because glycerol enters glycolysis at a later step and therefore delivers less energy. Moreover an additional ATP consumption is needed to drive glycerol uptake.<!--Quelle--> For the investigation of the basal transcription the fluorescence measurements, shown in Figure 10, is more interesting.<br> | ||
| + | In contrast to the other promoters characterized, like [http://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_S#Characterization_of_the_arabinose_promoter_PBAD P<sub>''BAD''</sub>] or [http://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_L#Characterization_of_the_lactose_promoter_Plac P<sub>''lac''</sub>], the fluorescence does not differ between the carbon sources used. This was expected in this case, because this operator is not enhanced by intracellular cAMP like the arabinoe or lactose promoter.</p> | ||
| + | <br> | ||
| + | [[File:Team-Bielefeld-Biosafeyt-System_TetORalive-TetOGFP-OD.jpg|600px|thumb|center|'''Figure 9:''' Characterization of the bacterial growth of the Biosafety-Strain K-12 ∆''alr'' ∆''dadX'' containing the plasmid <bbpart>BBa_K1172914</bbpart> with GFP (<bbpart>BBa_E0040</bbpart>) under the control of the ''tetO'' operator. The M9 medium was supplemented with 5 mM D-alanine. It could be demonstrated, that the bacteria grow faster on M9 minimal medium containing glucose than on M9 minimal medium with glycerol. ]] | ||
| − | [[File:Team-Bielefeld- | + | [[File:Team-Bielefeld-Biosafety-System-Tet2Flourescence.jpg|600px|thumb|center|'''Figure 10:''' Characterization of the fluorescence of the Biosafety-Strain K-12 ∆''alr'' ∆''dadX'' containing the plasmid <bbpart>BBa_K1172914</bbpart> with GFP (<bbpart>BBa_E0040</bbpart>) under control of the ''tetO'' operator. The Biosafety-Strain was cultivated on M9 minimal medium supplemented with 5 mM D-alanine..]] |
| + | <br> | ||
| + | <p align="justify"> | ||
| + | As for the other characterizations, the specific production rate was calculated to demonstrate in this case, that the carbon source does not influence the basal transcription, as shown in Figure 11. The specific production rates were calculated via equation (1) :</p><br> | ||
| + | [[File:IGEM Bielefeld 2013 Sepzifische Produktionsrate.png|600px|center|]] | ||
| + | <br> | ||
| + | <p align="justify"> | ||
| + | With the calculation of the specific production rate of GFP it can be demonstrated that the GFP synthesis rates does not differ between the cultivation on glucose and glycerol. The specific production rate show fluctuation on both cultivation, resulting in an up and down, so that there is can no obviously obvious be seen. This demonstrates that ''tetO'' operator is not enhanced by cAMP and confirms the results of the lactose and arabinose promoter, as they can be enhanced by cAMP and their basal transcription differs on glucose and glycerol.<br> | ||
| + | Moreover the specific production rate was calculated between every single measurement point, so the curve in Figure 11 is not smoothed and the fluctuations have to be ignored, as they do not stand for real fluctuations in the expression of GFP. They are caused by measurement variations in the growth curve and the fluorescence curve. And as neither of this curves are ideal, the fluctuations are the result. Nevertheless this graph shows the difference between the two carbon sources.</p><br> | ||
| + | |||
| + | [[File:Team-Bielefeld-Biosafeyt-System-TetORalive-specTetGFP.jpg|600px|thumb|center|'''Figure 11:''' Specific production rate of GFP expressed via the ''tetO'' operator in dependence of different carbon sources.]] | ||
| + | <br> | ||
==='''Characterization of the Biosafety-System TetOR alive'''=== | ==='''Characterization of the Biosafety-System TetOR alive'''=== | ||
| + | <br> | ||
| + | <p align="justify"> | ||
| + | The Biosafety-System ''TetOR alive'' was characterized on M9 minimal medium using glycerol as carbon source. As for the characterization of the pure ''tetO'' operator above, the bacterial growth and the fluorescence of GFP <bbpart>BBa_E0040</bbpart> was measured. Therefore, the wild type and the Biosafety-Strain ''E. coli'' K-12 ∆''alr'' ∆''dadX'' both containing the Biosafety-Plasmid <bbpart>BBa_K1172915</bbpart> were cultivated once with the induction of 1% L-rhamnose and once only on glycerol.<br> | ||
| + | |||
| + | It becomes obvious (Figure 12) that the bacteria, induced with 1 % L-rhamnose (red and black curve), grow obviously slower, than on pure glycerol (orange and blue curve). This might be attributed to the high metabolic burden encountered by the induced bacteria. The expression of the repressor TetR and the alanine racemase (Alr) simultaneously causes a high stress on the cells, so that they grow slower than the uninduced cells which express only GFP.<br> | ||
| − | [ | + | Comparing the bacterial growth with the fluorescence in Figure 15, it can be seen that the fluorescence of the Biosafety-Strain is much higher than the fluorescence of the wild type. This was also observed for the Biosafety-Strain [http://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_L#Characterization_of_the_Biosafety-System_Lac_of_Growth ''Lac of Growth''] and therefore the wild tpye is taken for further analysis of the Biosafety-Plasmid. While the Biosafety-Strain shows an decreasing fluorescence, the wild type shows an expected increase during cultivation. As observed for the other Biosafety-Systems, the fluorescence seems to follow the same trend than the bacterial growth. The uninduced cells show approximately an exponential rise of fluorescence, while in comparison the fluorescence of the induced bacteria increases only slowly.</p><br> |
| + | [[File:Team-Bielefeld-Biosafety-System-TetORalive-ODALL.jpg|600px|thumb|center|'''Figure 12:''' Characterization of the bacterial growth of the Biosafety-System ''TetOR alive'' on M9 minimal medium with glycerol. The Figure compares the wild type K-12 and the Biosafety-Strain K-12 ∆''alr'' ∆''dadX'' containing the Biosafety-Plasmid <bbpart>BBa_K1172915</bbpart> and the induction by 1% L-rhamnose to pure glycerol.]] | ||
| − | |||
| − | [[File:Team-Bielefeld-Biosafety-System-TetORalive- | + | [[File:Team-Bielefeld-Biosafety-System-TetORalive-FluorALL.jpg|600px|thumb|center|'''Figure 13:''' Characterization of the fluorescence of the Biosafety-System ''TetOR alive''. The Figure compares the wild type K-12 and the Biosafety-Strain K-12 ∆''alr'' ∆''dadX'' containing the Biosafety-Plasmid <bbpart>BBa_K1172915</bbpart> and the induction by 1% L-rhamnose to pure glycerol.]] |
| + | <br> | ||
| + | <p align="justify"> | ||
| + | From the data presented above, it cannot be determined if the expression of the repressor TetR does affect the transcription of GFP or not. Considering the wild type containing the Biosafety-System, the slower growth is a first indication that the repressor TetR and the alanine racemase (Alr) are highly expressed, but the growth of the bacteria shows nearly the same kinetics as the fluorescence. So it could be possible that the repressor does not affect the expression level of GFP under the control of the ''tetO'' operator. This becomes more clear by the calculation of the specific production rate of GFP by equation (1) . As shown in Figure 14 below the specific production rate does not really differ between the uninduced Biosafety-System and the Biosafety-System induced by 1% L-rhamnose.<br> | ||
| + | At the beginning the production of GFP in the presence of L-rhamnose (red curve) is lower than in its absence (orange curve), so that the expression of GFP seems to be repressed in the presence of L-rhamnose, but later one this changes and the specific production rate is constantly higher in the induced Biosafety-Strain. The fluctuation can be ignored, because the specific production rate of GFP was calculated between every single measurement point. The curve in Figure 14 is not smoothed , as they do not stand for real fluctuations in the expression of GFP. They are caused by measurement variations in the growth curve and the fluorescence curve. But this unexpected tendency that the production of GFP is lower when the bacteria are uninduced is obvious, so the Biosafety-System ''TetOR alive'' unfortunatly seems not to work. As the Biosafety-Systems [http://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_L#Conclusions ''Lac of Growth''] and [http://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_S#Conclusions ''AraCtive''] work, there must be a problem in the interaction of the TetR repressor with the ''tetO'' operator. This is discussed in the next section. | ||
| + | </p><br> | ||
| + | [[File:Team-Bielefeld-Biosafety-System-TetORalive-sepzProductALL.jpg|600px|thumb|center|'''Figure 14:''' Specific production rate of GFP for the Biosafety-System ''TetOR alive'', calculated via equation (1). The production rate of GFP of the uninduced bacteria fluctuates so that the Biosafety-System might not working as expected.]] | ||
| − | |||
| − | + | ==='''Conclusions'''=== | |
| + | <p align="justify"> | ||
| + | As mentioned above it seems that the Biosafety-System ''TetOR alive'' does not work as expected. This is also confirmed by Figure 15 showing the specific production rates of GFP after 7,5 hours of the induced Biosafety-System ''TetOR alive'' (red bar), the uninduced Biosafety-System (orange bar) and the pure ''tetO'' operator (<bbpart>BBa_K1172914</bbpart>). While induction does indeed results in a repression, the much lower expression from the pure ''tetO'' promoter/operator was not expected.</p><br> | ||
| + | [[File:Team-Bielefeld-Biosafety-System-TetORalive-Overview.jpg|600px|thumb|center|'''Figure 15:''' Comparison of the specific production rate of GFP. Shown are the induced (1% L-rhamnose) Biosafety-System ''TetOR alive'', the uninduced Biosafety-System ''TetOR alive'' and the second part of the Biosafety-System (''tetO'' - GFP only).]] | ||
| + | <br> | ||
| + | <p align="justify"> | ||
| + | As the Biosafety-Systems [http://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_L#Conclusions ''Lac of Growth''] and [http://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_S#Conclusions ''AraCtive''] show the same genetic construction, but differ only in the repressor and promoter, this cannot be an error of the construction. Also the [https://parts.igem.org/sequencing/part_analysis.cgi?part=BBa_K1172915 sequence analysis] show the expected sequence, so that this system should actually work. In the Partsregistry similar problems were reported, <!--link suche ich noch verzweifelt--> so this is might be due to an error in either of the parts, the repressor TetR <bbpart>BBa_C0040</bbpart> and/or the ''tetO'' operator <bbpart>BBa_R0040</bbpart>. So the Biosafety-Systems [http://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_L ''Lac of Growth''] and [http://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_S ''AraCtive''] work, while the Biosafety-System ''TetOR alive'' needs vigorous testing to identify and remove its problems. Nevertheless it would be worth to improve this part due to the tight shutdown and high activation of the Tet system which would be optimal for a biosafety system.</p><br> | ||
Latest revision as of 14:06, 29 October 2013
Biosafety-System TetOR alive (TetR)
The Biosafety-System TetOR alive BBa_K1172915 is an improvement of the BioBrick BBa_K914014 by replacing the first promoter into the rhamnose promoter PRha, integration of the alanine racemase BBa_K1172901 and utilization of the repressor TetR to regulate the transcription of the Barnase behind the tetO promoter. Because this system is known for a tight repression and a fast activation, it is expected that bacteria containing this Biosafety-System are dead or alive...
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 1156
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 1080
Illegal BamHI site found at 1782 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 1198
Illegal AgeI site found at 1498 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 955
Illegal BsaI.rc site found at 2774
Biosafety system TetOR alive
Combining the genes described above with the Biosafety-Strain K-12 ∆alr ∆dadX results in a powerful device, allowing us to control the bacterial cell division. The control of the bacterial growth is possible either active or passive. Active by inducing the tetO operator with tetracycline and passive by the induction of L-rhamnose. The passive control makes it possible to control the bacterial cell division in a defined closed environment, like the MFC, by continuously adding L-rhamnose to the medium. As shown in Figure 1 below, this leads to an expression of the essential alanine racemase (alr) and the TetR repressor, so that the expression of the RNase Ba is repressed.
In the event that bacteria exit the defined environment of the MFC or L-rhamnose is not added to the medium any more, both the expression of the alanine racemase (Alr) and the TetR repressor decrease, so that the expression of the toxic RNase Ba (Barnase) begins. The cleavage of the intracellular RNA by the Barnase and the lack of synthesized D-alanine, caused by the repressed alanine racemase inhibit the cell division. Through this it can be secured that the bacteria can only grow in the defined area or the device of choice respectively.

Results
Characterization of the tetracycline tetO operator
First, the tetO operator was characterized to get a first impression of its basal transcription rate. Therefore, the bacterial growth was investigated using the unrepressed tetO operator on different carbon source using M9 minimal medium with either glucose or glycerol. The transcription rate was identified by fluorescence measurement of GFP BBa_E0040 behind the tetO operator BBa_R0040 using the BioBrick BBa_K1172914.
As shown in Figure 9 below, the bacteria grew slightly better on glucose then on glycerol. This is due to glucose being the better energy source of these two, because glycerol enters glycolysis at a later step and therefore delivers less energy. Moreover an additional ATP consumption is needed to drive glycerol uptake. For the investigation of the basal transcription the fluorescence measurements, shown in Figure 10, is more interesting.
In contrast to the other promoters characterized, like [http://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_S#Characterization_of_the_arabinose_promoter_PBAD PBAD] or [http://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_L#Characterization_of_the_lactose_promoter_Plac Plac], the fluorescence does not differ between the carbon sources used. This was expected in this case, because this operator is not enhanced by intracellular cAMP like the arabinoe or lactose promoter.


As for the other characterizations, the specific production rate was calculated to demonstrate in this case, that the carbon source does not influence the basal transcription, as shown in Figure 11. The specific production rates were calculated via equation (1) :
With the calculation of the specific production rate of GFP it can be demonstrated that the GFP synthesis rates does not differ between the cultivation on glucose and glycerol. The specific production rate show fluctuation on both cultivation, resulting in an up and down, so that there is can no obviously obvious be seen. This demonstrates that tetO operator is not enhanced by cAMP and confirms the results of the lactose and arabinose promoter, as they can be enhanced by cAMP and their basal transcription differs on glucose and glycerol.
Moreover the specific production rate was calculated between every single measurement point, so the curve in Figure 11 is not smoothed and the fluctuations have to be ignored, as they do not stand for real fluctuations in the expression of GFP. They are caused by measurement variations in the growth curve and the fluorescence curve. And as neither of this curves are ideal, the fluctuations are the result. Nevertheless this graph shows the difference between the two carbon sources.
Characterization of the Biosafety-System TetOR alive
The Biosafety-System TetOR alive was characterized on M9 minimal medium using glycerol as carbon source. As for the characterization of the pure tetO operator above, the bacterial growth and the fluorescence of GFP BBa_E0040 was measured. Therefore, the wild type and the Biosafety-Strain E. coli K-12 ∆alr ∆dadX both containing the Biosafety-Plasmid BBa_K1172915 were cultivated once with the induction of 1% L-rhamnose and once only on glycerol.
It becomes obvious (Figure 12) that the bacteria, induced with 1 % L-rhamnose (red and black curve), grow obviously slower, than on pure glycerol (orange and blue curve). This might be attributed to the high metabolic burden encountered by the induced bacteria. The expression of the repressor TetR and the alanine racemase (Alr) simultaneously causes a high stress on the cells, so that they grow slower than the uninduced cells which express only GFP.
Comparing the bacterial growth with the fluorescence in Figure 15, it can be seen that the fluorescence of the Biosafety-Strain is much higher than the fluorescence of the wild type. This was also observed for the Biosafety-Strain [http://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_L#Characterization_of_the_Biosafety-System_Lac_of_Growth Lac of Growth] and therefore the wild tpye is taken for further analysis of the Biosafety-Plasmid. While the Biosafety-Strain shows an decreasing fluorescence, the wild type shows an expected increase during cultivation. As observed for the other Biosafety-Systems, the fluorescence seems to follow the same trend than the bacterial growth. The uninduced cells show approximately an exponential rise of fluorescence, while in comparison the fluorescence of the induced bacteria increases only slowly.


From the data presented above, it cannot be determined if the expression of the repressor TetR does affect the transcription of GFP or not. Considering the wild type containing the Biosafety-System, the slower growth is a first indication that the repressor TetR and the alanine racemase (Alr) are highly expressed, but the growth of the bacteria shows nearly the same kinetics as the fluorescence. So it could be possible that the repressor does not affect the expression level of GFP under the control of the tetO operator. This becomes more clear by the calculation of the specific production rate of GFP by equation (1) . As shown in Figure 14 below the specific production rate does not really differ between the uninduced Biosafety-System and the Biosafety-System induced by 1% L-rhamnose.
At the beginning the production of GFP in the presence of L-rhamnose (red curve) is lower than in its absence (orange curve), so that the expression of GFP seems to be repressed in the presence of L-rhamnose, but later one this changes and the specific production rate is constantly higher in the induced Biosafety-Strain. The fluctuation can be ignored, because the specific production rate of GFP was calculated between every single measurement point. The curve in Figure 14 is not smoothed , as they do not stand for real fluctuations in the expression of GFP. They are caused by measurement variations in the growth curve and the fluorescence curve. But this unexpected tendency that the production of GFP is lower when the bacteria are uninduced is obvious, so the Biosafety-System TetOR alive unfortunatly seems not to work. As the Biosafety-Systems [http://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_L#Conclusions Lac of Growth] and [http://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_S#Conclusions AraCtive] work, there must be a problem in the interaction of the TetR repressor with the tetO operator. This is discussed in the next section.
Conclusions
As mentioned above it seems that the Biosafety-System TetOR alive does not work as expected. This is also confirmed by Figure 15 showing the specific production rates of GFP after 7,5 hours of the induced Biosafety-System TetOR alive (red bar), the uninduced Biosafety-System (orange bar) and the pure tetO operator (BBa_K1172914). While induction does indeed results in a repression, the much lower expression from the pure tetO promoter/operator was not expected.
File:Team-Bielefeld-Biosafety-System-TetORalive-Overview.jpg
As the Biosafety-Systems [http://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_L#Conclusions Lac of Growth] and [http://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_S#Conclusions AraCtive] show the same genetic construction, but differ only in the repressor and promoter, this cannot be an error of the construction. Also the sequence analysis show the expected sequence, so that this system should actually work. In the Partsregistry similar problems were reported, so this is might be due to an error in either of the parts, the repressor TetR BBa_C0040 and/or the tetO operator BBa_R0040. So the Biosafety-Systems [http://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_L Lac of Growth] and [http://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_S AraCtive] work, while the Biosafety-System TetOR alive needs vigorous testing to identify and remove its problems. Nevertheless it would be worth to improve this part due to the tight shutdown and high activation of the Tet system which would be optimal for a biosafety system.