Difference between revisions of "Part:BBa K525234"
(→Intracellular localisation) |
(→Purification) |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 61: | Line 61: | ||
===Expression in ''E. coli''=== | ===Expression in ''E. coli''=== | ||
| − | The CspB | + | The CspB gene was fused with a monomeric RFP ([https://parts.igem.org/Part:BBa_E1010 BBa_E1010]) using [http://2011.igem.org/Team:Bielefeld-Germany/Protocols#Gibson_assembly Gibson assembly] for characterization. |
| − | The CspB|mRFP fusion protein was overexpressed in E. coli KRX after induction of T7 polymerase by supplementation of 0,1 % L-rhamnose using the [http://2011.igem.org/Team:Bielefeld-Germany/Protocols/Downstream-processing#Expression_of_S-layer_genes_in_E._coli autinduction protocol] from | + | The CspB|mRFP fusion protein was overexpressed in ''E. coli'' KRX after induction of T7 polymerase by supplementation of 0,1 % L-rhamnose using the [http://2011.igem.org/Team:Bielefeld-Germany/Protocols/Downstream-processing#Expression_of_S-layer_genes_in_E._coli autinduction protocol] from Promega. |
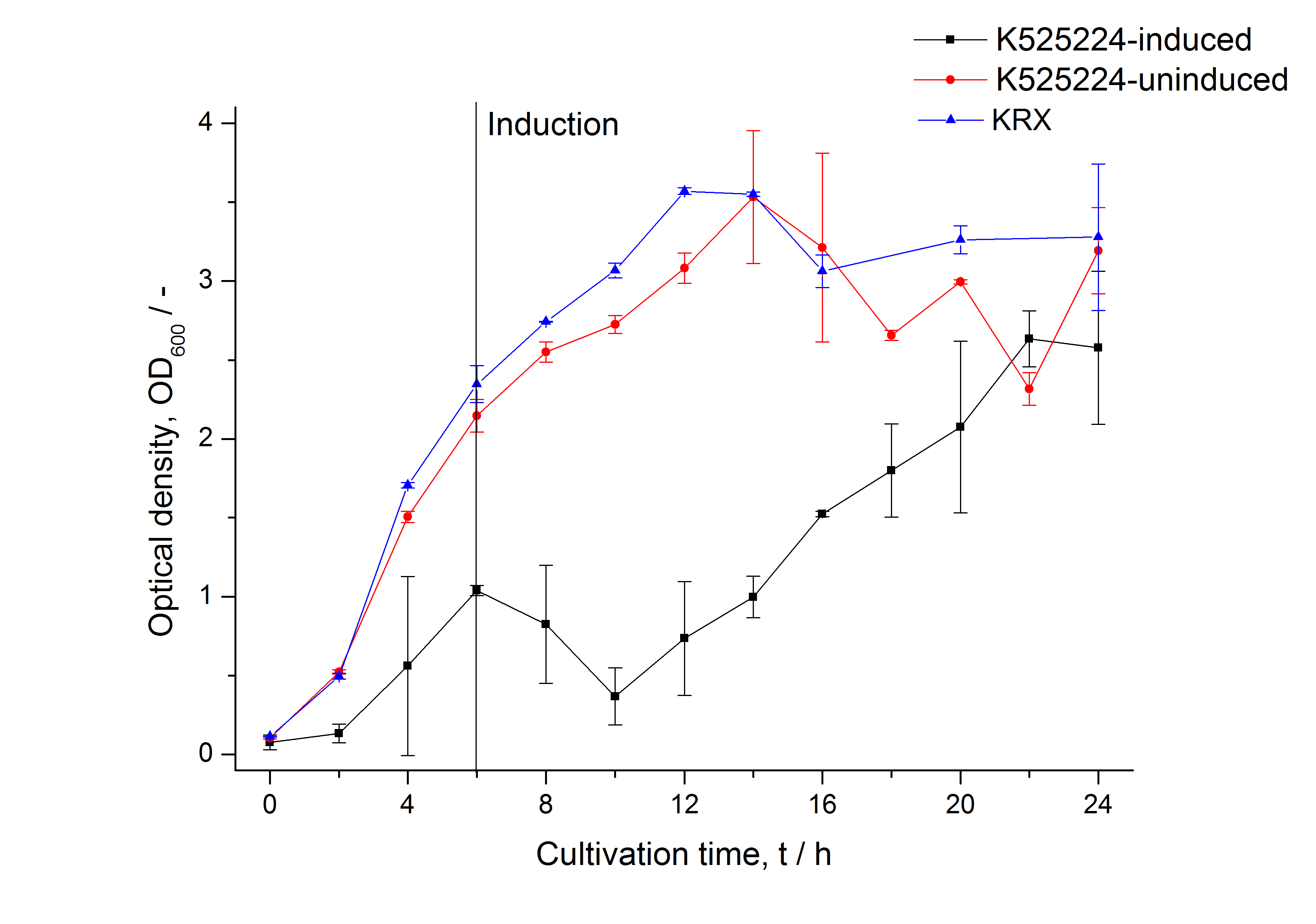
[[Image:Bielefeld_2011_CH4_Growthcurve.png|600px|center|thumb| '''Figure 1: Growthcurve of ''E. coli'' KRX expressing the fusion protein of CspB and mRFP with and without induction, cultivated at 37 °C in autoinduction medium with, respectively, without inductor. A curve depicting KRX wildtype is shown for comparsion. After induction at approximately 6 h the OD<sub>600</sub> of the induced K525224 visibly drops when compared to the uninduced culture. While the induced culture grow significantly slower than KRX wildtype the uninduced seems to be unaffected.''']] | [[Image:Bielefeld_2011_CH4_Growthcurve.png|600px|center|thumb| '''Figure 1: Growthcurve of ''E. coli'' KRX expressing the fusion protein of CspB and mRFP with and without induction, cultivated at 37 °C in autoinduction medium with, respectively, without inductor. A curve depicting KRX wildtype is shown for comparsion. After induction at approximately 6 h the OD<sub>600</sub> of the induced K525224 visibly drops when compared to the uninduced culture. While the induced culture grow significantly slower than KRX wildtype the uninduced seems to be unaffected.''']] | ||
| Line 71: | Line 71: | ||
===Intracellular and localisation=== | ===Intracellular and localisation=== | ||
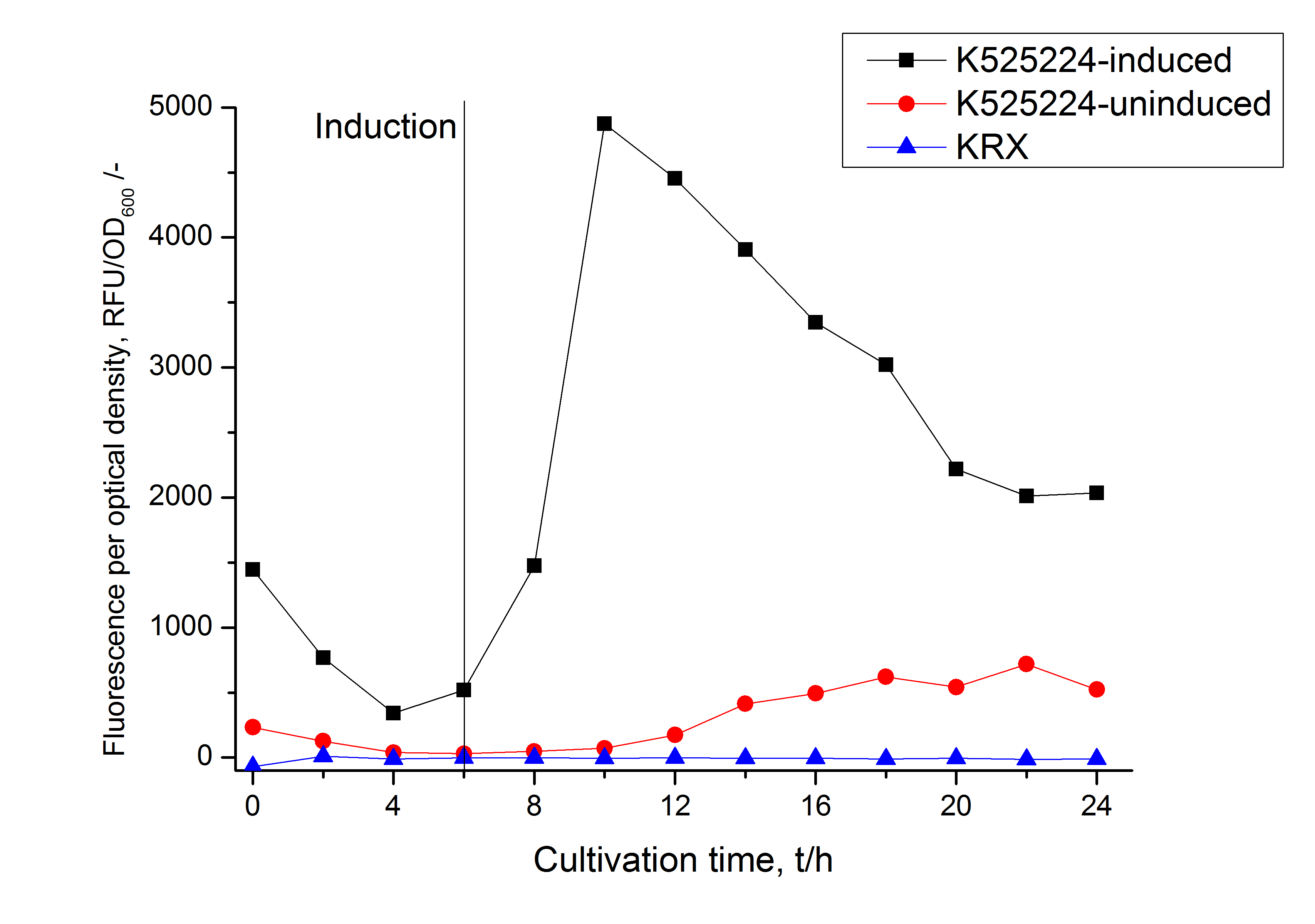
| − | After a cultivation time of 18 h the mRFP|CspB fusion protein has to be localized in ''E. coli'' KRX. Therefor a part of the produced biomass was mechanically disrupted and the resulting lysate was | + | After a cultivation time of 18 h the mRFP|CspB fusion protein has to be localized in ''E. coli'' KRX. Therefor a part of the produced biomass was mechanically disrupted and the resulting lysate was washed with ddH<sub>2</sub>O. Then the lysate was treated with ionic, nonionic and zwitterionic detergents to release the mRFP|CspB out of the membranes, if it intigrates. From the other part of the cells the periplasm was detached by using a osmotic shock. The existance of fluorescene in the periplasm fraction, showed in fig. 3, indicates that ''C. halotolerans'' TAT-signal sequence is at least in part functional in ''E. coli'' KRX. |
| − | Specific for K525224 fused with mRFP is the proportional to the mRFP fusion proteins of [https://parts.igem.org/Part:BBa_K525222 K525222] and [https://parts.igem.org/Part:BBa_K525223 K525223] high fluorescence in the culture supernatant. This indicates that the fusion protein is secreted into the periplasm via the TAT-pathway and partly | + | Specific for K525224 fused with mRFP is the proportional to the mRFP fusion proteins of [https://parts.igem.org/Part:BBa_K525222 K525222] and [https://parts.igem.org/Part:BBa_K525223 K525223] high fluorescence in the culture supernatant. This indicates that the fusion protein is secreted into the periplasm via the TAT-pathway and partly released into the culture medium. Because there is no known release pathway for S-layer proteins in ''E. coli'' the periplasm might burst in consequence of the overexpression. |
The fluorescence in all cultivation fractions plus the fluorescence in the lysis und wash fraction shows that the fusion protein is water soluble and doesn't sediment during centrifugation. | The fluorescence in all cultivation fractions plus the fluorescence in the lysis und wash fraction shows that the fusion protein is water soluble and doesn't sediment during centrifugation. | ||
| Line 80: | Line 80: | ||
| − | [[Image:Bielefeld 2011 CH4 Purification.png|700px|thumb|center| '''Figure 3: Fluorescence progression of the mRFP [https://parts.igem.org/Part:BBa_E1010 (BBa_E1010)]/CspB fusion protein initiating with the cultivation fractions up to the detergent fractions of the seperate denaturations. Cultivations were carried out in autoinduction medium at 37 ˚C. The cells were mechanically disrupted and the resulting biomass was | + | [[Image:Bielefeld 2011 CH4 Purification.png|700px|thumb|center| '''Figure 3: Fluorescence progression of the mRFP [https://parts.igem.org/Part:BBa_E1010 (BBa_E1010)]/CspB fusion protein initiating with the cultivation fractions up to the detergent fractions of the seperate denaturations. Cultivations were carried out in autoinduction medium at 37 ˚C. The cells were mechanically disrupted and the resulting biomass was washed with ddH<sub>2</sub>O and resuspended in the respective detergent. The used detergent acronyms stand for: SDS = 10 % sodium dodecyl sulfate; UTU = 7 M urea and 3 M thiourea; U = 10 M urea; NLS = 10 % n-lauroyl sarcosine; 2 % CHAPS = 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate.''']] |
<!-- --> | <!-- --> | ||
===Purification=== | ===Purification=== | ||
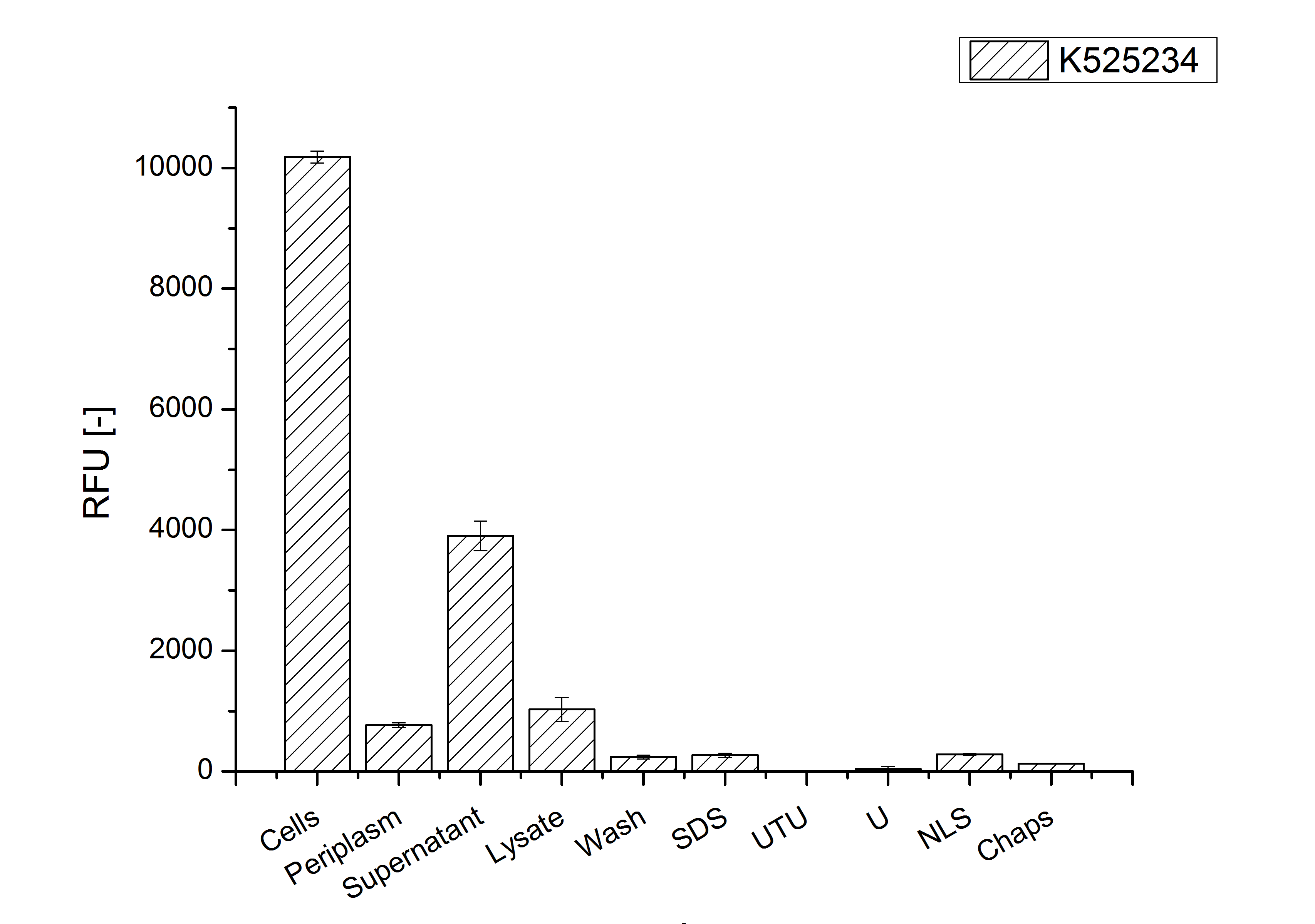
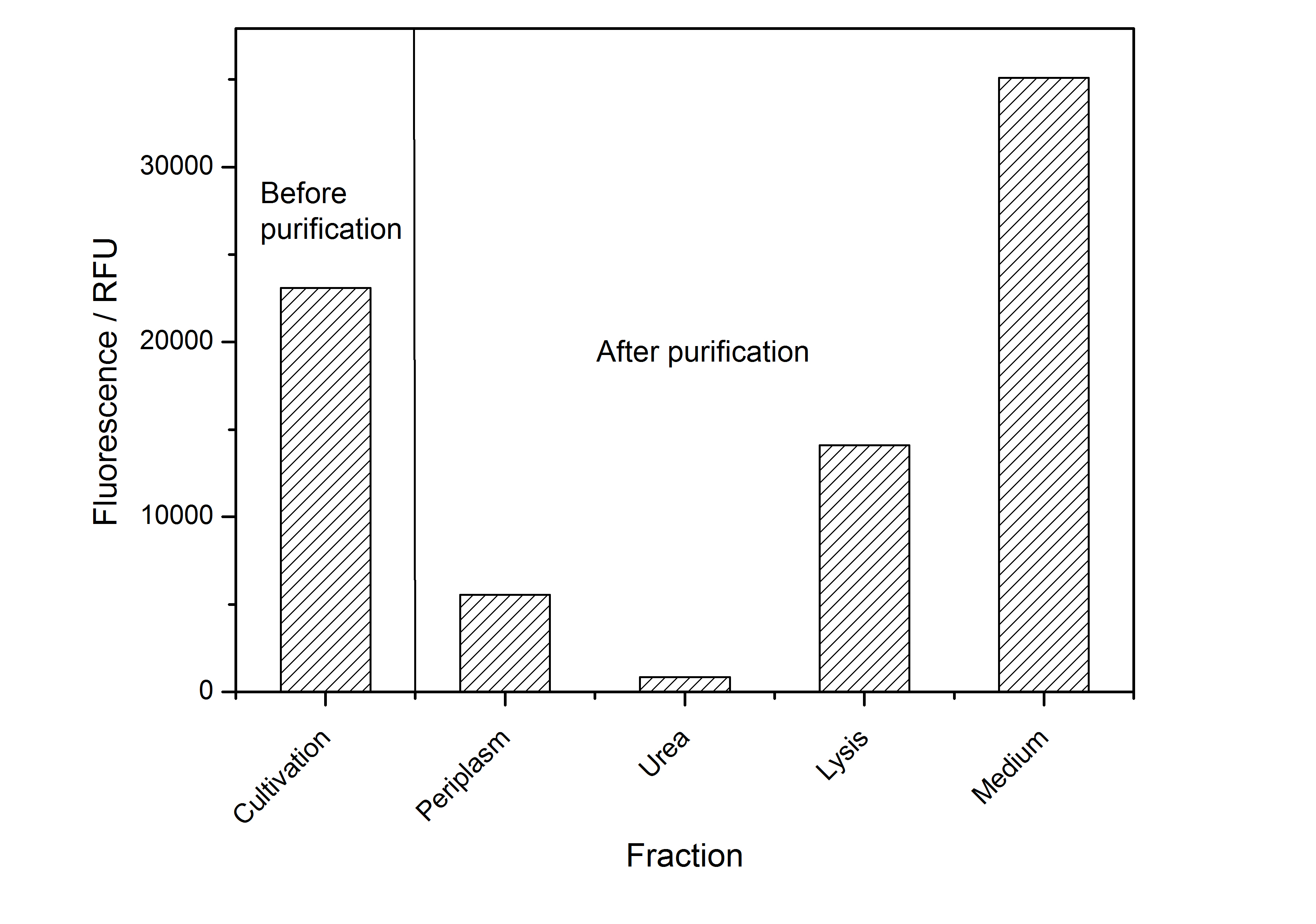
| − | After the localisation of the S-layer protein in ''E. coli'', different methods for purification were tested. The results of these methods are shown in fig. | + | After the localisation of the S-layer protein in ''E. coli'', different methods for purification were tested. The results of these methods are shown in fig. 4. Fig. 4 shows, that the CspB protein does not form inclusion bodies in ''E. coli'' and most of the protein is transported out of the cell into the periplasm and a lot of protein is even secreted into the medium (all fractions were concentrated by filtration and precipitation, respectively). The secretion into the culture medium is very interesting because the purification is much faster (no cell disruption necessary). |
| − | [[Image:Bielefeld-Germany2011-CH4-purificationfractions.jpg|700px|center|thumb|'''Fig. | + | [[Image:Bielefeld-Germany2011-CH4-purificationfractions.jpg|700px|center|thumb|'''Fig. 4: Fluorescence of collected fractions of different methods to release and concentrate <partinfo>K525234</partinfo> protein from a cultivation in ''E. coli''. ''']] |
| − | The highest fluorescence could be obtained by a precipitation with ammonium sulfate of the culture supernatant followed by an ultrafiltration with a 300 kDa membrane and a diafiltration with a 50 kDa membrane. The diafiltration was against a binding buffer for an anion exchange chromatography (25 mM sodium acetate, 25 mM sodium chloride) with pH 6, due to the theoretical pI of <partinfo>k525234</partinfo>. The fluorescence of the collected fractions of the following anion exchange chromatography are shown in fig. | + | The highest fluorescence could be obtained by a precipitation with ammonium sulfate of the culture supernatant followed by an ultrafiltration with a 300 kDa membrane and a diafiltration with a 50 kDa membrane. The diafiltration was against a binding buffer for an anion exchange chromatography (25 mM sodium acetate, 25 mM sodium chloride) with pH 6, due to the theoretical pI of <partinfo>k525234</partinfo>. The fluorescence of the collected fractions of the following anion exchange chromatography are shown in fig. 5. |
| − | [[Image:Bielefeld-Germany2011-CH4_Med_IEX.jpg|700px|center|thumb|'''Fig. | + | [[Image:Bielefeld-Germany2011-CH4_Med_IEX.jpg|700px|center|thumb|'''Fig. 5: Fluorescence of collected fractions of an anion exchange chromatography of <partinfo>K525234</partinfo> after concentration from the culture supernatant. ''']] |
| − | The binding conditions are well chosen because nearly all of the protein binds to the column. The protein is eluted from the column with rising sodium chloride concentrations. The highest fluorescence is in the elution fraction with 400 mM sodium chloride. | + | The binding conditions are well chosen because nearly all of the protein binds to the column. The protein is eluted from the column with rising sodium chloride concentrations. The highest fluorescence is in the elution fraction with 400 mM sodium chloride. 600 mM sodium chloride elutes all of the S-layer fusion proteins. |
Latest revision as of 08:07, 28 October 2011
Fusion Protein of mRFP, S-layer cspB from Corynebacterium halotolerans with TAT-sequence, PT7, RBS
S-layers (crystalline bacterial surface layer) are crystal-like layers consisting of multiple protein monomers and can be found in various (archae-)bacteria. They constitute the outermost part of the cell wall. Especially their ability for self-assembly into distinct geometries is of scientific interest. At phase boundaries, in solutions and on a variety of surfaces they form different lattice structures. The geometry and arrangement is determined by the C-terminal self assembly-domain, which is specific for each S-layer protein. The most common lattice geometries are oblique, square and hexagonal. By modifying the characteristics of the S-layer through combination with functional groups and protein domains as well as their defined position and orientation to eachother (determined by the S-layer geometry) it is possible to realize various practical applications ([http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full Sleytr et al., 2007]).
Usage and Biology
S-layer proteins can be used as scaffold for nanobiotechnological applications and devices by e.g. fusing the S-layer's self-assembly domain to other functional protein domains. It is possible to coat surfaces and liposomes with S-layers. A big advantage of S-layers: after expressing in E. coli and purification, the nanobiotechnological system is cell-free. This enhances the biological security of a device.
This fluorescent S-layer fusion protein is used to characterize purification methods and the S-layer's ability to self-assemble on surfaces.
Important parameters
| Experiment | Characteristic | Result |
|---|---|---|
| Expression (E. coli) | Localisation | Inclusion body |
| Compatibility | E. coli KRX and BL21(DE3) | |
| Inductor for expression | T7 polymerase | |
| Specific growth rate (un-/induced) | 0.251 h-1 / 0.157 h-1 | |
| Doubling time (un-/induced) | 2.76 h / 4.42 h | |
| Purification | Molecular weight | 79.2 kDa |
| Theoretical pI | 4.54 | |
| Excitation / emission | 584 / 607 nm |
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal PstI site found at 799
Illegal PstI site found at 1125 - 12INCOMPATIBLE WITH RFC[12]Illegal PstI site found at 799
Illegal PstI site found at 1125 - 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 1876
Illegal XhoI site found at 1332 - 23INCOMPATIBLE WITH RFC[23]Illegal PstI site found at 799
Illegal PstI site found at 1125 - 25INCOMPATIBLE WITH RFC[25]Illegal PstI site found at 799
Illegal PstI site found at 1125
Illegal NgoMIV site found at 999
Illegal NgoMIV site found at 2088
Illegal AgeI site found at 87
Illegal AgeI site found at 680
Illegal AgeI site found at 792
Illegal AgeI site found at 990
Illegal AgeI site found at 1231
Illegal AgeI site found at 1278 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 1680
Illegal BsaI.rc site found at 987
Illegal BsaI.rc site found at 1365
Illegal BsaI.rc site found at 1767
Expression in E. coli
The CspB gene was fused with a monomeric RFP (BBa_E1010) using [http://2011.igem.org/Team:Bielefeld-Germany/Protocols#Gibson_assembly Gibson assembly] for characterization.
The CspB|mRFP fusion protein was overexpressed in E. coli KRX after induction of T7 polymerase by supplementation of 0,1 % L-rhamnose using the [http://2011.igem.org/Team:Bielefeld-Germany/Protocols/Downstream-processing#Expression_of_S-layer_genes_in_E._coli autinduction protocol] from Promega.
Intracellular and localisation
After a cultivation time of 18 h the mRFP|CspB fusion protein has to be localized in E. coli KRX. Therefor a part of the produced biomass was mechanically disrupted and the resulting lysate was washed with ddH2O. Then the lysate was treated with ionic, nonionic and zwitterionic detergents to release the mRFP|CspB out of the membranes, if it intigrates. From the other part of the cells the periplasm was detached by using a osmotic shock. The existance of fluorescene in the periplasm fraction, showed in fig. 3, indicates that C. halotolerans TAT-signal sequence is at least in part functional in E. coli KRX.
Specific for K525224 fused with mRFP is the proportional to the mRFP fusion proteins of K525222 and K525223 high fluorescence in the culture supernatant. This indicates that the fusion protein is secreted into the periplasm via the TAT-pathway and partly released into the culture medium. Because there is no known release pathway for S-layer proteins in E. coli the periplasm might burst in consequence of the overexpression.
The fluorescence in all cultivation fractions plus the fluorescence in the lysis und wash fraction shows that the fusion protein is water soluble and doesn't sediment during centrifugation.
The absence of fluotrescence indicates that the expressed fusion protein doesn't form inclusion bodies during cultivation.
Purification
After the localisation of the S-layer protein in E. coli, different methods for purification were tested. The results of these methods are shown in fig. 4. Fig. 4 shows, that the CspB protein does not form inclusion bodies in E. coli and most of the protein is transported out of the cell into the periplasm and a lot of protein is even secreted into the medium (all fractions were concentrated by filtration and precipitation, respectively). The secretion into the culture medium is very interesting because the purification is much faster (no cell disruption necessary).
The highest fluorescence could be obtained by a precipitation with ammonium sulfate of the culture supernatant followed by an ultrafiltration with a 300 kDa membrane and a diafiltration with a 50 kDa membrane. The diafiltration was against a binding buffer for an anion exchange chromatography (25 mM sodium acetate, 25 mM sodium chloride) with pH 6, due to the theoretical pI of BBa_K525234. The fluorescence of the collected fractions of the following anion exchange chromatography are shown in fig. 5.
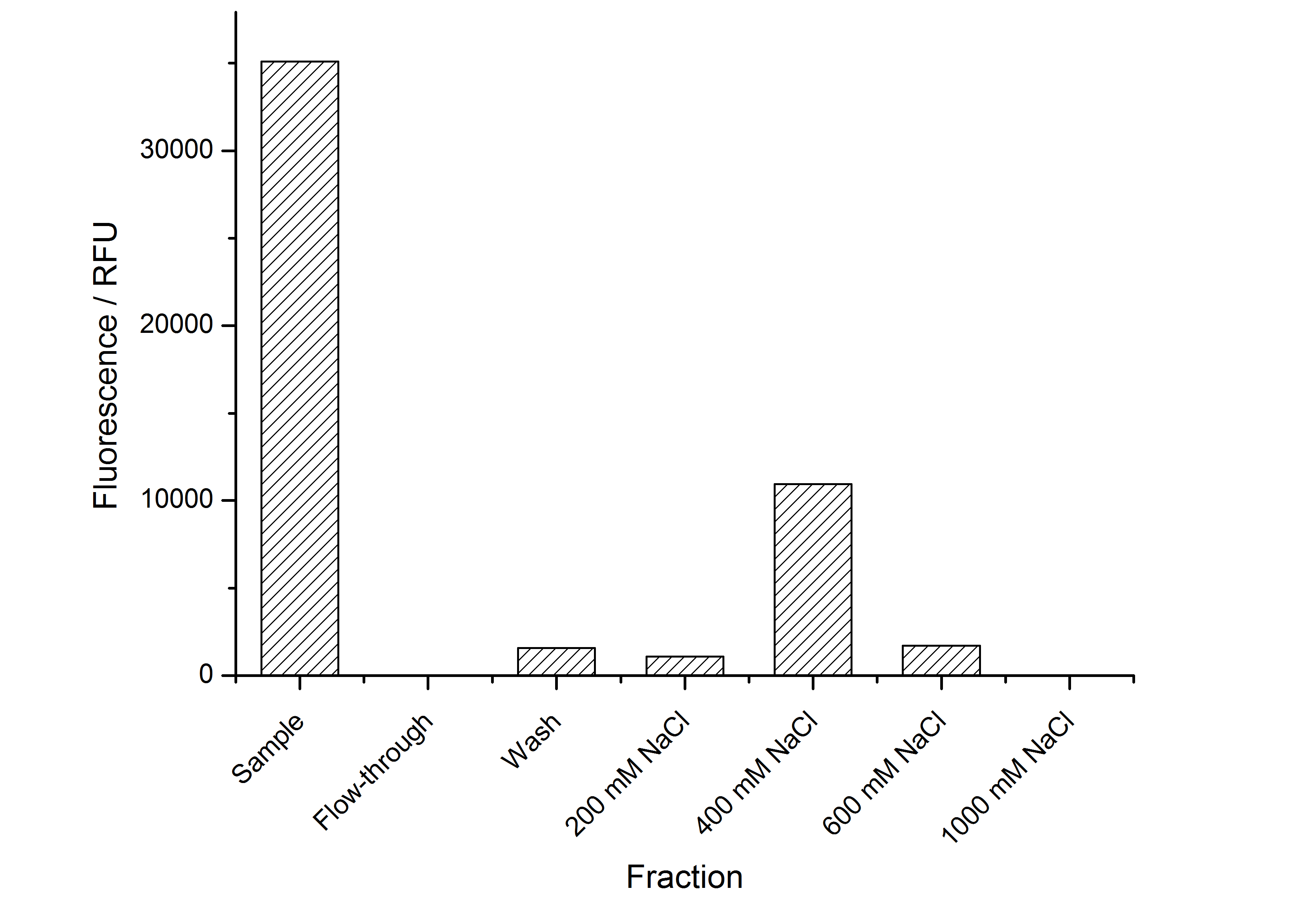
The binding conditions are well chosen because nearly all of the protein binds to the column. The protein is eluted from the column with rising sodium chloride concentrations. The highest fluorescence is in the elution fraction with 400 mM sodium chloride. 600 mM sodium chloride elutes all of the S-layer fusion proteins.