Part:BBa_K4247008
Contents
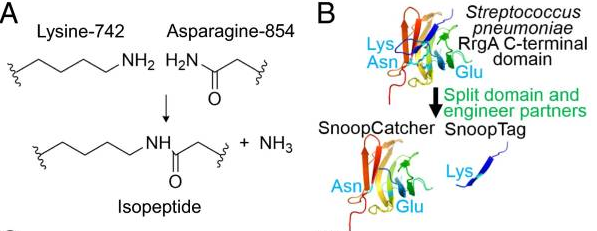
SnoopTag
This part codes for SnoopTag, which is a short peptide that together with the basic part BBa_K4247009 (SnoopCatcher) enables to link proteins through a covalent bond formation between the Tag and Catcher. It has been used extensively to form polyproteins in a spontaneous reaction (but can be supported by SnoopLigase).
Usage and Biology
The Tag-Catcher system is a protein conjugation tool that enables the formation of an irreversible isopeptide bond between these two components. A covalent peptide/protein pair was developed to enable spontaneous isopeptide bond formation between peptide tags. This system was developed based on Gram-positive surface protein, the pilus adhesin RrgA of S. pneumoniae. The D4 domain of this protein is stabilised by an isopeptide forming between a lysine (K742) and an asparagine (N854). This domain was split into a scaffold protein called SnoopCatcher and a 12-residue peptide termed SnoopTag, which can spontaneously form a covalent isopeptide bond upon mixing. The initiation, extension, and release steps use mild conditions, independent of redox state, and therefore should be applicable to a wide range of proteins according to the original paper.
Characterization
Addition of SnoopTag to Minispidroin_NT-2rep-CT_N-6His
Aim - To attach the sequence coding for SnoopTag to the protein minispidroin_NT-2rep-CT_N-6His (BBa_K4247007) via PCR and produce the proteins.
Results -
Conclusion - We can clearly see the protein with the SnoopTag (34.6kDa) since the band is slightly higher than that of the protein without the SnoopTag (30kDa)
Protein purification by IMAC
Aim - To purify the protein by IMAC (immobilized metal ion chromatography) using Ni-NTA resin.
Results - Ni-NTA resin was added to the soluble fraction of the lysate and allowed to incubate for 1h under shaking conditions. Then, it was centrifuged to collect the resin with the proteins and the supernatant was collected as flowthrough. Then, the resin was washed twice and eluted twice. We would expect to see a band at around 34.6kDa since that is the molecular weight of the protein.
Conclusion - Hence, it is clear that the presence of the SnoopTag does not affect the purification of the protein since it is eluted in high amounts, good purity and without much losses in the flowthrough or washes.
Addition of SnoopTag to Minispidroin_NT-4rep-CT_N-6His
Aim - To attach the sequence coding for SnoopTag to the protein minispidroin_NT-4rep-CT_N-6His (BBa_K4247012) via PCR and produce the proteins.
Results -
Conclusion- We can clearly see the protein with the SnoopTag (41.8 kDa) since the band is slightly higher than that of the protein without the SnoopTag (40.3kDa).
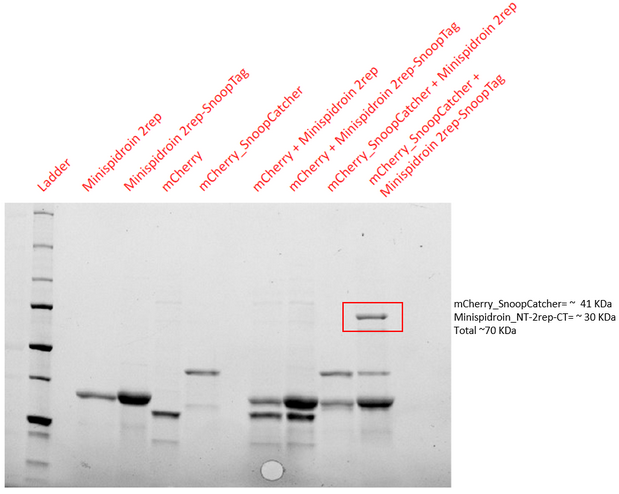
Validation of SnoopTag’s function
Aim - To demonstrate the spontaneous isopeptide bond formation between our minispidroin protein displaying the SnoopTag and mCherry_SnoopCatcher or mfp151_SnoopCatcher.
Results - The proteins were allowed to interact with each other by mixing equal amounts of both proteins. The reaction was carried out at 25°C for 40 minutes and the pH of the reaction was maintained at 8 using pH adjusted PBS. After 40 minutes, the reaction was stopped by adding SDS sample buffer. Then, we performed an SDS-PAGE and a Western blot to visualize the results. We would expect to see a band whose molecular weight is the sum of the molecular weights of both proteins to indicate that both proteins are bound to each via an isopeptide bond between the SnoopTag and the SnoopCatcher. We see such a band only in the reaction where mCherry_SnoopCatcher or mfp151_SnoopCatcher and Minispidroin 2rep with a SnoopTag were allowed to interact. The fact that we don't see this band in any of the controls confirms that the proteins were able to bind to each other solely due to the SnoopTag-Catcher system.
Conclusion - The SnoopTag enabled the Minispidroin 2rep to bind to mCherry_SnoopCatcher or mfp151_SnoopCatcher and hence, the function of the SnoopTag-Catcher system was validated.
Modelling SnoopTag-Catcher effect on minispidroin
We modelled the structure of the SnoopTag-Catcher system with the goal of determining whether they would affect the structure of our silk protein parts. For this purpose we therefore used AlphaFold 2.0 and saw that the termini of our silk proteins (key for fiber formation) were not affected.
Here the Tag, Catcher, repetitive part of minispidroin and homodimers (two termini binding) can be observed, and their structure is still what expected based on the N and C termini models of minispidroin (parts BBa_K4247000, BBa_K4247002).
| None |