Part:BBa_K4040003
Intracellular Domain of CD3 zeta chain
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
T-cell surface glycoprotein CD3 zeta chain is part of the TCR-CD3 complex present on T-lymphocyte cell surface that plays an essential role in adaptive immune response. When antigen presenting cells (APCs) activate T-cell receptor (TCR), TCR-mediated signals are transmitted across the cell membrane by the CD3 chains CD3D, CD3E, CD3G and CD3Z. All CD3 chains contain immunoreceptor tyrosine-based activation motifs (ITAMs) in their cytoplasmic domain. Upon TCR engagement, these motifs become phosphorylated by Src family protein tyrosine kinases LCK and FYN, resulting in the activation of downstream signaling pathways [1,2].
CD3Z ITAMs phosphorylation creates multiple docking sites for the protein kinase ZAP70 leading to ZAP70 phosphorylation and its conversion into a catalytically active enzyme [2].
It plays an important role in intrathymic T-cell differentiation. Additionally, participates in the activity-dependent synapse formation of retinal ganglion cells (RGCs) in both the retina and dorsal lateral geniculate nucleus (dLGN) (By similarity).
Background and detail description
Used in our project
The synthetic receptors were constructed to contain an scFv derived from an antibody recognizing the virus spike protein, CR3022, which has been reported to bind with the receptor-binding domain of the SARS-CoV-2 S glycoprotein with high affinity, and the CD8 transmembrane domain present in the aCD19 CAR for T cells. For the cytoplasmic domains, we used the common g subunit of Fc receptors (CARg), MEGF10 (CARMEGF10), MERTK (CARMERTK) and CD3z (CARz) in our study. These cytoplasmic domains are capable of promoting phagocytosis by macrophages[3]. More details and experimental results can be found in CAR-CD3 zeta(BBa_K4040017)
Used in the construction of CAR-Macrophages cells
Previous study has engineered phagocytes that recognize and ingest targets through specific antibody-mediated interactions. This strategy can be directed towards multiple extracellular ligands(CD19 and CD22) and can be used with several intracellular signaling domains that contain ITAM motifs (Megf10, FcRV, and CD3z). Previous work has suggested that spatial segregation between Src-family kinases and an inhibitory phosphatase, driven by receptor ligation, is sufficient to trigger signaling by the T cell receptor (Davis and van der Merwe, 2006; James and Vale, 2012) and FcR(Freeman et al., 2016). The CAR-Ps that we have developed may similarly convert receptor-ligand binding into receptor phosphorylation of ITAM domains through partitioning of kinases and phosphatases at the membrane-membrane interface.
Thus, although using CAR-Ps to enhance cross presentation of cancer antigen is an intriguing future avenue, such a strategy would likely require more optimization of the dendritic cell subset employed or the CAR-P receptor itself. Previous study has demonstrated that the CAR approach is transferrable to biological processes beyond T cell activation and that the expression of an engineered receptor in phagocytic cells is sufficient to promote specific engulfment and elimination of cancer cells.
Previous study has found that a chimeric adenoviral vector overcame the inherent resistance of primary human macrophages to genetic manipulation and imparted a sustained proinflammatory (M1) phenotype. CAR macrophages (CARMs) demonstrated antigenspecific phagocytosis and tumor clearance in vitro. In two solid tumor xenograft mouse models, a single infusion of human CARMs decreased tumor burden and prolonged overall survival. Characterization of CARM activity showed that CARMs expressed proinflammatory cytokines and chemokines, converted bystander M2 macrophages to M1, upregulated antigen presentation machinery, recruited and presented antigen to T cells and resisted the effects of immunosuppressive cytokines. In humanized mouse models, CARMs were further shown to induce a proinflammatory tumor microenvironment and boost antitumor T cell activity[5].
CAR-P is a successful strategy for directing macrophages towards cancer targets, and can initiate whole cell eating and trogocytosis leading to cancer cell elimination(Figure 2)[4].

CAR-M phagocytosis was an active process requiring Syk, non-muscle myosin IIA and actin polymerization, similarly to Fc receptor-mediated ADCP[5].
Colocalization and antitumor acti vity of CARM were evaluated by immunohistochemical analysis of explanted lungs, revealing the presence of multiple meta static tumor nests in this model (Fig. 3a), with a significant reduction in metastatic tumor burden after CARM treatment (Fig. 3b)[5].

Macrophage phenotype is plastic and can change in response to cytokines, pathogen-associated molecular patterns, metabolic cues, cell–cell interactions and tissuespecific signals[6]. CARMs maintained a proinflammatory phenotype within the human TME.
Using the humanized TME mouse model, the effect of CARMs on the surrounding TME was interrogated at singlecell resolution. Adoptively transferred macrophages were removed from analysis by excluding all human male cells. The human TME grouped into two large clusters (cluster 0 and cluster 1) and one small cluster of cells that was excluded from analysis (cluster 2). Cluster 0 was enriched in the CARMtreated arm (86% CAR, 14% UTD), whereas cluster 1 was enriched in the UTDtreated arm (71% UTD, 29% CAR) (Fig. 4a). Gene expression analysis of cluster 0 revealed an enrichment of proinflammatory genes such as MHCII and TNF , demonstrating that CARMs remodeled the TME toward a proinflammatory state (Fig. 4b)[5].

CAR-Ms induced pro-inflammatory pathways such as interferon signaling, TH1 pathway and iNOS signaling in M2 macrophages (Fig. 5)[5].
Additionally, CARMs induced activation and maturation markers in immature human dendritic cells (Fig. 6a) and directly induced the recruitment of both resting and activated human T cells in an in vitro chemotaxis assay (Fig. 6b). Finally, having shown that CARMs can exert a dominant effect on surrounding immune cells, we demonstrated that CARMs maintained their antitumor activity in the presence of human M2 macrophages (Fig. 6c).

To test CARM crosspresentation of intracellular tumor antigens ingested during phagocytosis of whole tumor cells, we generated SKOV3 expressing NYESO1 only (SKOVNY), SKOV3 expressing HLAA201 and NYESO1 (SKOVA201NY) and CARMs from an HLAA201+ donor. TRAC knockout antiNYESO1 TCR T cells were incubated with CARMs (macrophage control), SKOVNY (tumor control), SKOVA2NY (positive control) or CARMs that were fed SKOVNY for 48 h. CD8+ antiNYESO1 T cells were activated (as determined by CD69 induction and production of IFNγ) by CARMs that ingested SKOVNY (Fig. 6). AntiNYESO1 T cells were not activated by SKOVNY or CARMs alone, demonstrating that macrophages were able to crosspresent tumorderived antigens after phagocytosis, suggesting that CARMs might lead to epitope spreading.

CAR-M eradicated SKOV3, a HER2+ ovarian cancer cell line, in a dose and time-dependent manner (Fig. 8a,b).

In T cells, phosphorylated ITAMs in CD3z bind to tandem SH2 domains (tSH2) in the kinase ZAP70. Zap70 is not expressed in macrophages, but Syk, a phagocytic signaling effector and tSH2 domain containing protein, is expressed at high levels (Andreu et al., 2017). Previous work suggested that Syk kinase can also bind to the CD3z ITAMs (Bu et al., 1995), indicating that the CAR-T may promote engulfment through a similar mechanism as CAR-PFcRV. To quantitatively compare the interaction between SyktSH2and CD3z or FcRV in a membrane proximal system recapitulating physiological geometry, a study used a liposome-based assay (Figure 2 [Hui and Vale, 2014]). In this system, His10CD3z and His10-Lck (the kinase that phosphorylates CD3z) are bound to a liposome via NiNTA-lipids and the binding of labeled tandem SH2 domains to phosphorylated CD3z was measured using fluorescence quenching. Their results show that Syk-tSH2 binds the CD3z and FcRV with comparable affinity (~15 nM and ~30 nM respectively).
Collectively, these results demonstrate that the TCR CD3z chain can promote phagocytosis in a CAR-P, likely through the recruitment of Syk kinase[4].

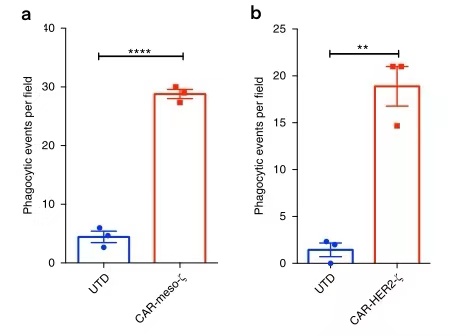
Previous study has generated CAR-Ms targeting the solid tumor antigens mesothelin or HER2 and demonstrated phagocytosis of antigen-positive target cells (Fig.10a,b)[5]. Together, these data demonstrated that CD3ζ based CARs can direct anti-tumor phagocytic activity and provided support for subsequent efforts to translate this platform to primary human macrophages.
References
[1]Barber EK, Dasgupta JD, Schlossman SF, Trevillyan JM, Rudd CE. The CD4 and CD8 antigens are coupled to a protein-tyrosine kinase (p56lck) that phosphorylates the CD3 complex. Proc Natl Acad Sci U S A. 1989 May;86(9):3277-81. doi: 10.1073/pnas.86.9.3277. PMID: 2470098; PMCID: PMC287114.
[2]Iwashima M, Irving BA, van Oers NS, Chan AC, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994 Feb 25;263(5150):1136-9. doi: 10.1126/science.7509083. PMID: 7509083.
[3]Fu W, Lei C, Ma Z, Qian K, Li T, Zhao J, Hu S. CAR Macrophages for SARS-CoV-2 Immunotherapy. Front Immunol. 2021 Jul 23;12:669103. doi: 10.3389/fimmu.2021.669103. PMID: 34367135; PMCID: PMC8343226.
[4]Morrissey MA, Williamson AP, Steinbach AM, Roberts EW, Kern N, Headley MB, Vale RD. Chimeric antigen receptors that trigger phagocytosis. Elife. 2018 Jun 4;7:e36688. doi: 10.7554/eLife.36688. PMID: 29862966; PMCID: PMC6008046.
[5]Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, Schmierer M, Gabrusiewicz K, Anderson NR, Petty NE, Cummins KD, Shen F, Shan X, Veliz K, Blouch K, Yashiro-Ohtani Y, Kenderian SS, Kim MY, O'Connor RS, Wallace SR, Kozlowski MS, Marchione DM, Shestov M, Garcia BA, June CH, Gill S. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020 Aug;38(8):947-953. doi: 10.1038/s41587-020-0462-y. Epub 2020 Mar 23. PMID: 32361713; PMCID: PMC7883632.
[6]Mosser, D. M. & Edwards, J. P . Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008).
| None |