Part:BBa_K3895005
Keratinase kerBlMKU3
kerBlMKU3 is a serine keratinase originally secreted by Bacillus licheniformis MKU3. It can work at 37°C for 48 h in nutrient broth or on feather medium [1].
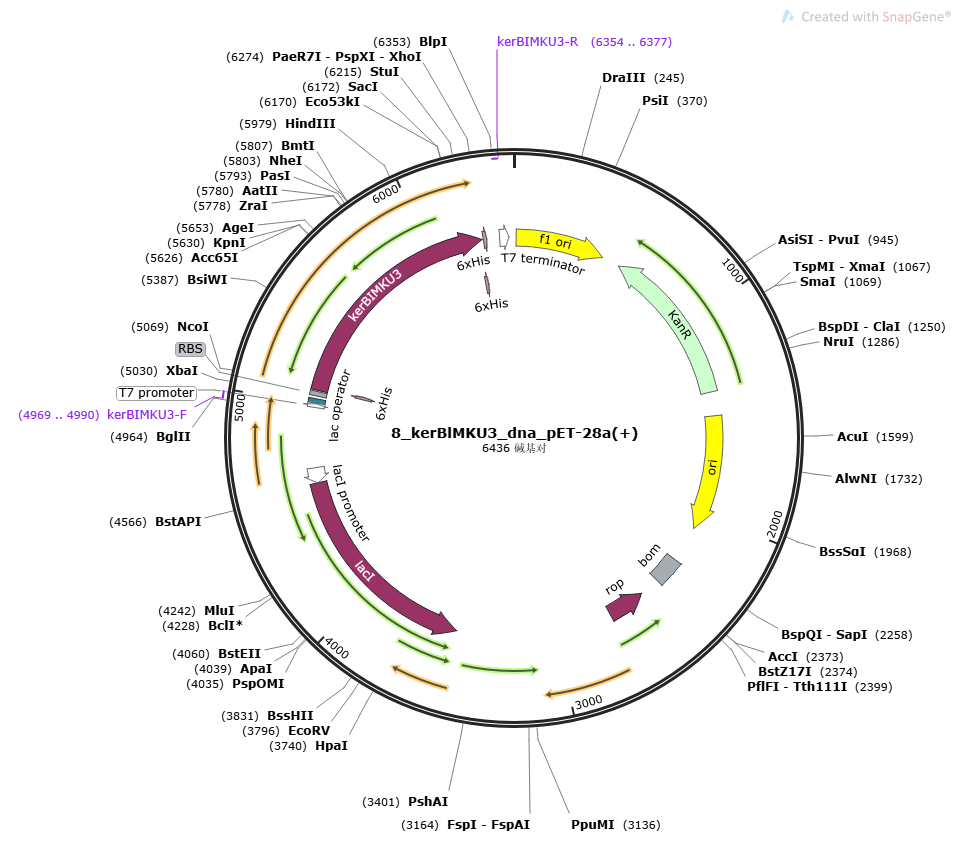
6x His-tags were added to both sides of the sequence for purification, and constructed into PET-28a(+) (BBa_K3895009). The length of kerBlMKU3 is 1202 bp.
| Name of keratinase | IPTG concentration/mM | Temperature/°C | Time/h |
|---|---|---|---|
| KerBIMKU3 | 0.4 | 37 | 4 |
Modeling
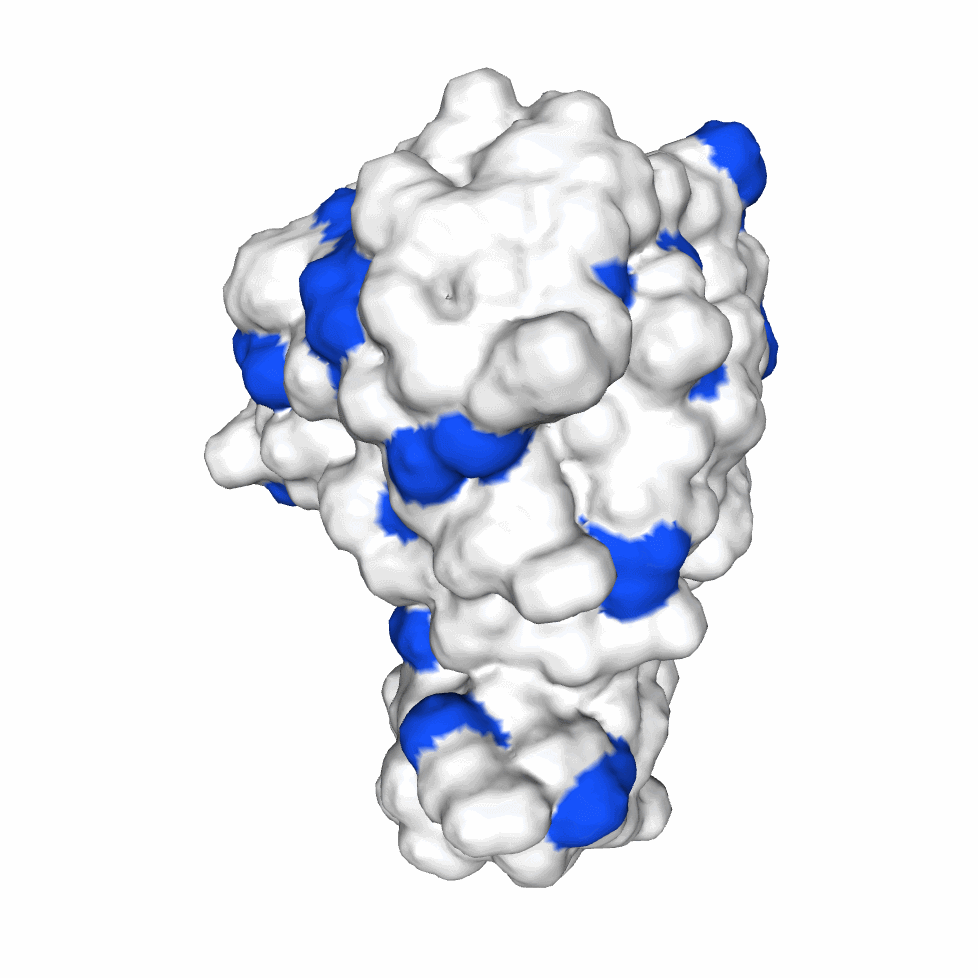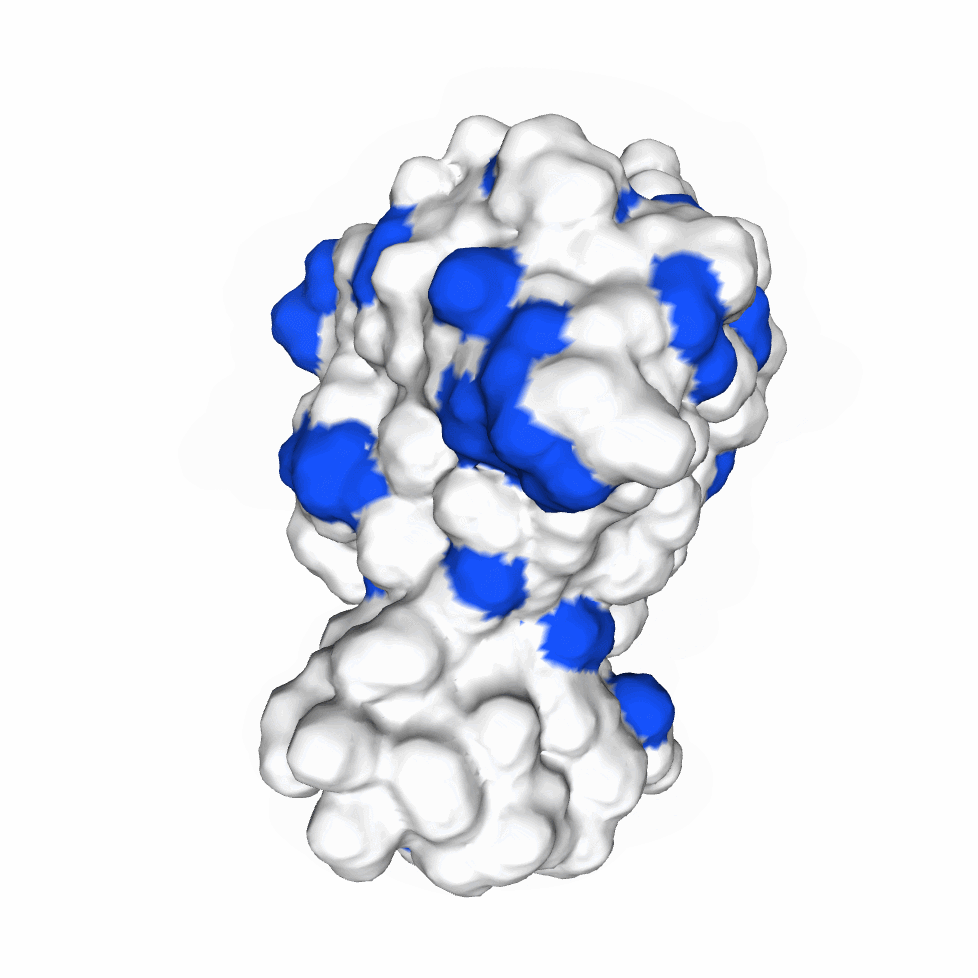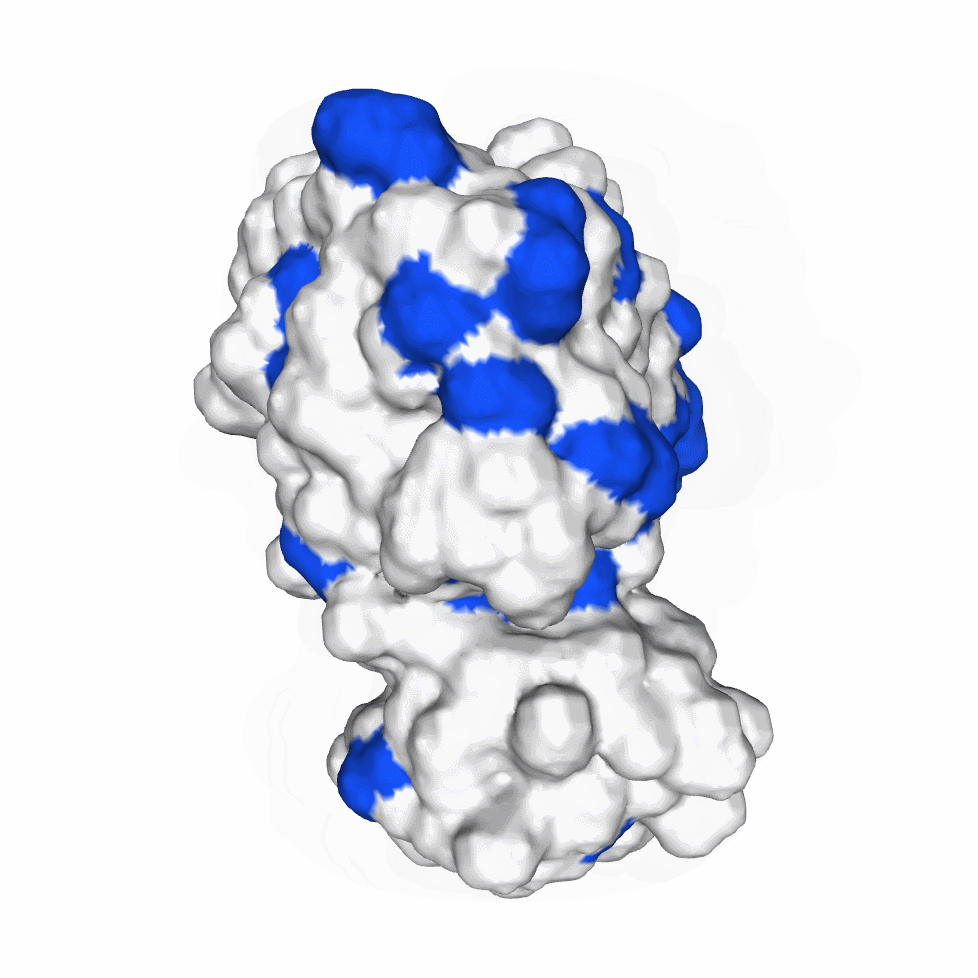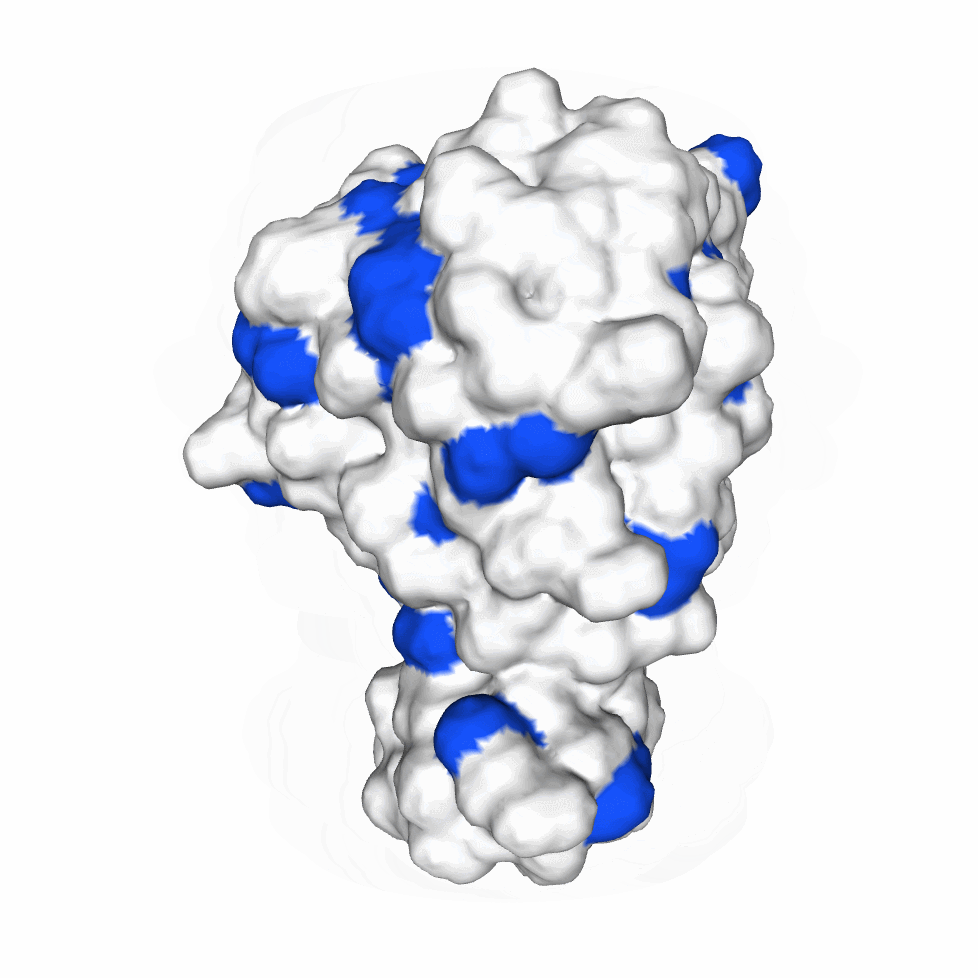
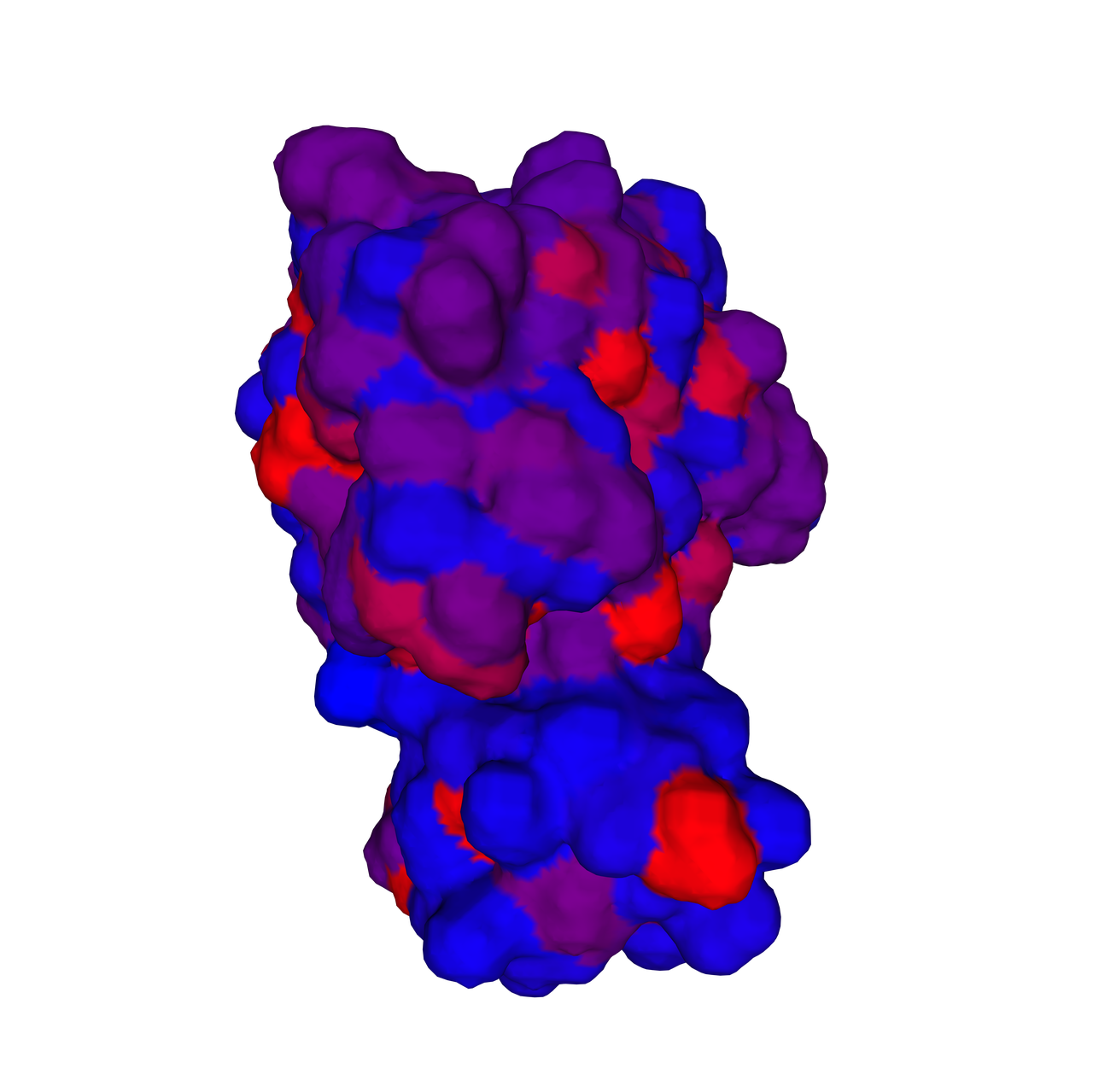
The biological activity of a protein is not only determined by the primary structure of the protein molecule but also closely related to its specific spatial structure. Elucidating the process of protein folding in functional and structural details will be of great significance to evaluate their keratinotic functions. According to the amino acid sequences in the enzyme active sites and associated catalytic mechanisms, proteases can be classified into seven broad groups: serine, cysteine, threonine, aspartic and glutamic proteases, metalloproteases and asparagine peptide lyases. According to the nature of their active site, keratinases belong to serine- and metalloproteases or serine metalloproteases. Moreover, the enzymatic degradation of keratin is a multistage process that requires the following steps: adsorption of the keratinases to the surface of macromolecule by electrostatic and hydrophobic interactions, followed by catalytic action [2]. Hence we used Swiss Model to predict their hydrophobic region and serine groups.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 733
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 583
- 1000COMPATIBLE WITH RFC[1000]
References
Radha, S., & Gunasekaran, P. (2007). Cloning and expression of keratinase gene in Bacillus megateriumand optimization of fermentation conditions for the production of keratinase by recombinant strain. Journal of Applied Microbiology, 103(4), 1301–1310. https://doi.org/10.1111/j.1365-2672.2007.03372.x
Vidmar, Beti, and Maša Vodovnik. “Microbial Keratinases: Enzymes with Promising Biotechnological Applications.” Food technology and biotechnology vol. 56,3 (2018): 312-328. doi:10.17113/ftb.56.03.18.5658
| None |