Part:BBa_K849001
Farnesyl-Pyrophosphate-Synthase from Yeast
This is the Farnesyl-Pyrophosphate-Synthase from Saccharomyces cerevisiae which produces Farnesyl-Pyrophosphate from Isoprenyl Pyrophosphate and Dimethylallyl Pyrophosphate. It is encoded by the gen ERG20. We inserted some calm mutations to avoid conflicts with the Cloning Standard 10.
For further information: http://aem.asm.org/content/75/17/5536.short
Kenro Tokuhiro, Masayoshi Muramatsu,Chikara Ohto, Toshiya Kawaguchi,Shusei Obata,Nobuhiko Muramoto,Masana Hirai, Haruo Takahashi, Akihiko Kondo, Eiji Sakuradani and Sakayu Shimizu: Overproduction of Geranylgeraniol by Metabolically Engineered Saccharomyces cerevisiae Appl. Environ. Microbiol. 2009, 75(17):5536.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
KEYSTONE 2022 Characterization
We engineered E.coli Strain DH5a(tnaA-) to heterologously express ERG20 to test the yield of santalene.
Santalene production in E.coli DH5α
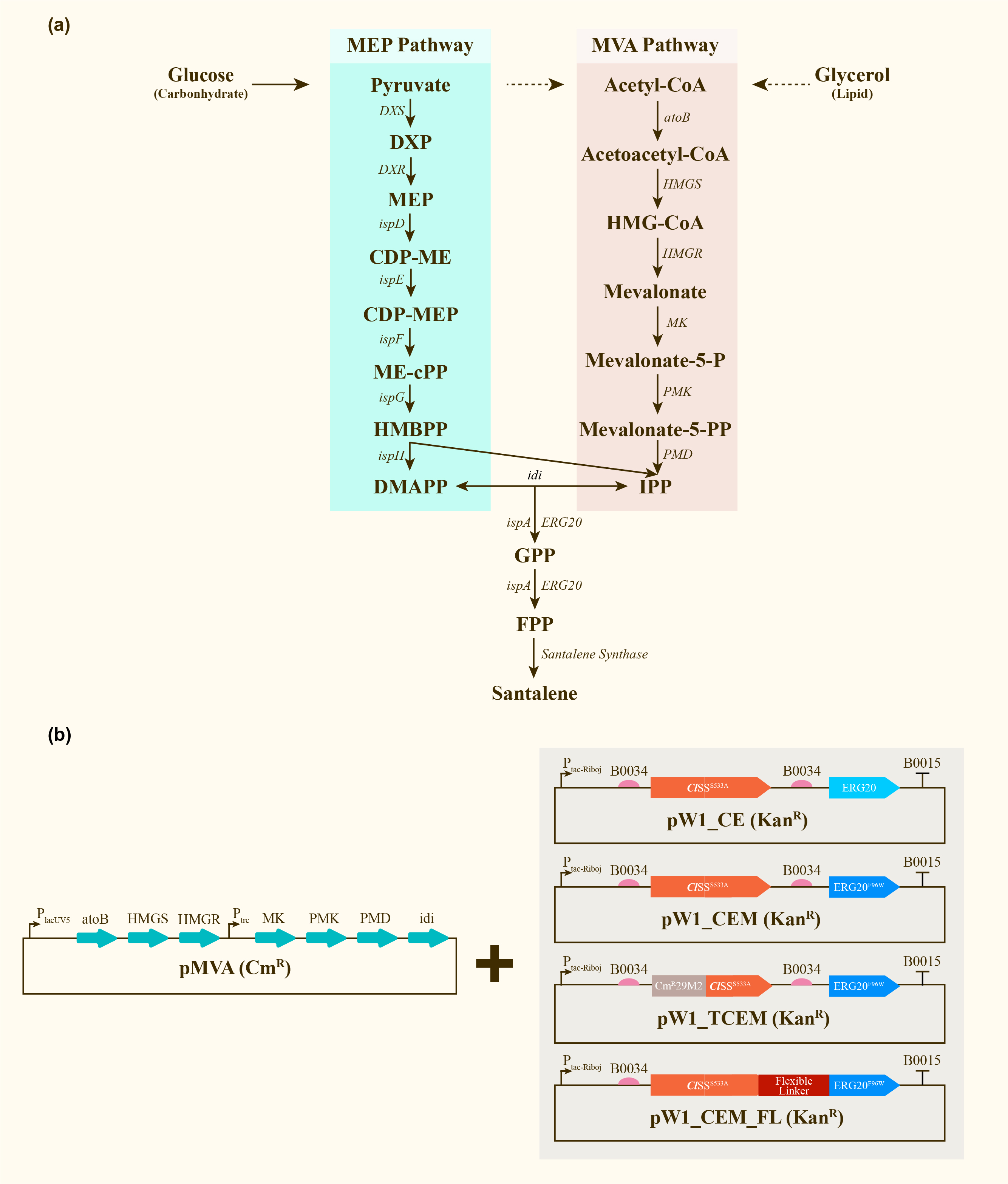
After engineering, E. coli could utilize both MEP pathway and MVA pathway for the universal precursors isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP), then synthesize santalene with the help of FPP Synthase (FPPS) and santalene synthase (SS). Except heterologously expressed MVA pathway and ERG20 of Saccharomyces cerevisiae and santalene synthase of Clausena lansium (ClSS), several modifications upon ERG20 or ClSS by amino acid mutation, binding to a hydrophillic tag and the construction of fusion protein were tested for the higher yield of santalene. Therefore, with the help of the co-transformation of pMVA plasmid with various pW1 plasmids, including pW1_CE, pW1_CEM, pW1_TCEM and pW1_CEM_FL, different strains like CE, CEM, TCEM, CEM_FL were successfully constructed (Figure 1). The complete pathway we designed for producing santalene in E. coli is illustrated in Figure 1.
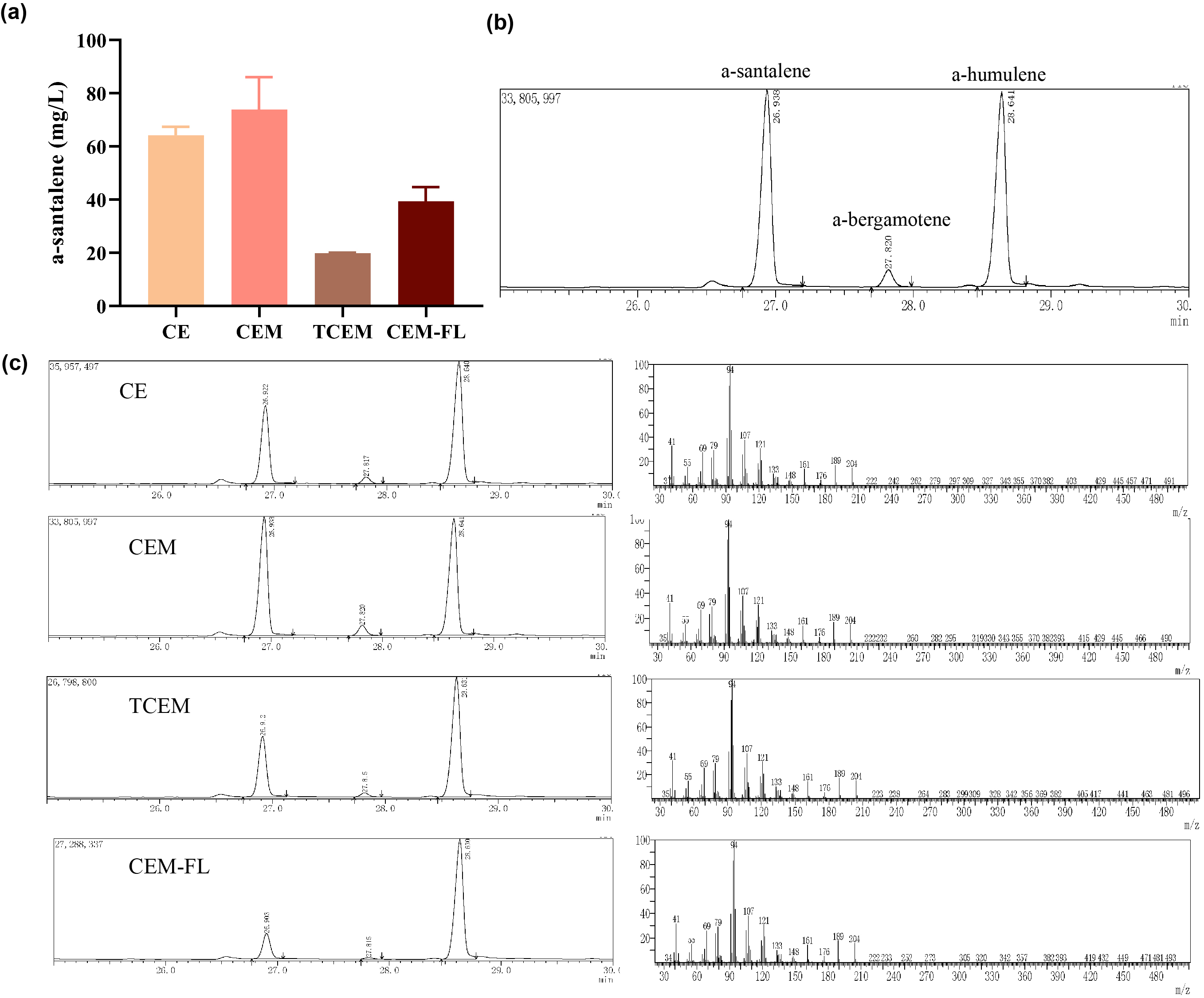
Afterwards, the various engineering of E.coli DH5α ∆TnaA mentioned above were used for santalene production. After rapid centrifugation, the supernatant of dodecane was spiked with with 0.475 g/L a-humulene as an internal standard, and then injected into GC/MS for verification of α-santalene production. It turned out that all samples from four strains appeared a significant peak at the retention time of 26-27 min, and various peak area of different samples exhibited santalene production with differing levels, indicating the general success of E. coli engineering. It can be concluded that the E. coli strain CEM (with pW1_CEM plasmid) produces the maximal level of α-santalene compared to other strains (73.93 mg/L). Furthermore, our study elucidates that the mutation of 96th amino acid into tryptophan could increase the yield of α-santalene by about 20%, substantiating the prominent performance of ERG20F96W in enhancing the supply of FPP and α-santalene production in E. coli (Figure 2).
| None |
