Part:BBa_K4195005
RhaS
An activator of L-rhamnose inducible promoter.
Biology
Among the L-rhamnose inducible promoter, the rhaPBAD is regulated by two activators, RhaR and RhaS. In the presence of L-rhamnose, RhaR binds to the rhaPRS promoter and activates the production of RhaR and RhaS. RhaS together with L-rhamnose in turn binds to the rhaPBAD and the rhaPT promoter and activates the transcription of the structural genes(1).
Usage
We expressed the rhaS gene additionally before the promoter pRha (BBa_K914003) using the constitutive promoter BBa_J23106 in order to obtain stronger expression. BBa_E0030 was chosen as the reportor protein to measure the expression intensity of L-rhamnose inducible promoter. Different sub parts were assembled into pSB4K5 plasmid backbone using Gibson assembly to get the composite part BBa_K4195109 (Fig. 1). The Gibson assembly mixture was transformed into E. coli DH5α & E. coli BL21(DE3), and the positive transformants were confirmed by kanamycin, colony PCR and sequencing.
Fig. 1 Gene circuit of BBa_K4195109.
Characterization
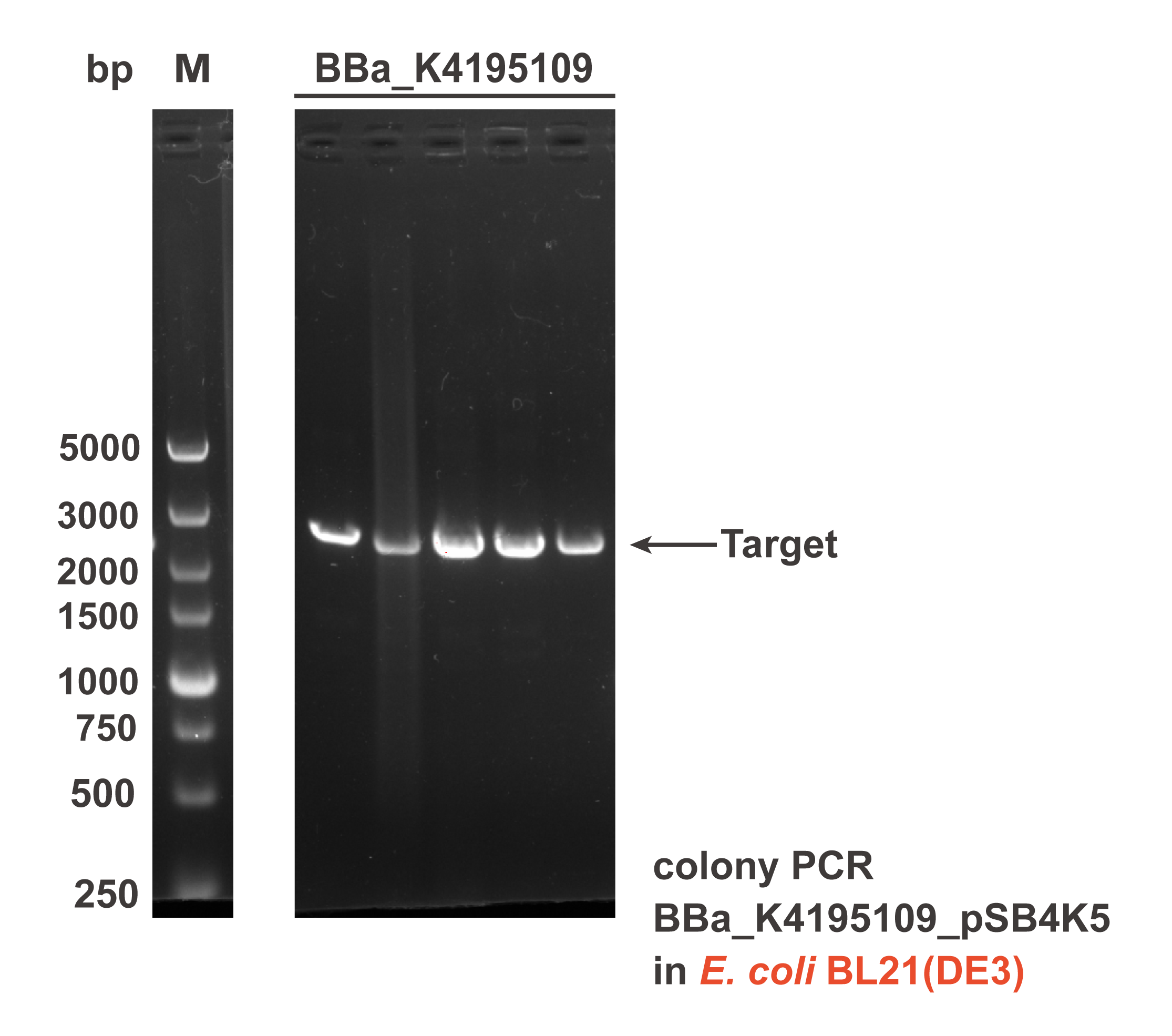
When we were building this circuit, colony PCR was used to certify the plasmid was correct. We got the target fragment-2331bp (lane K4195109).
Fig. 2 The result of colony PCR. Plasmid pSB4K5.
Then, the colony with the corrected sequence was cultivated and induced to measure the expression intensity of EYFP compared with BBa_K4195191. Both the composite parts were induced under L-rhamnose whose final concentration was 0.1 mg/mL and the same volume sterile water was added as the control. The fluorescence intensity and OD600 was measured in 2 h after induction. The gene circuit with rhaS can produce higher fluorescent signal obviously although there exists little expression leakage. (Fig. 3)
Fig. 3 The comparison of normalized fluorescence intensity between BBa_K4195109 and BBa_K4195191.
What’s more, we also set a series of concentration gradients to compared different inducers’ effects on the improved L-rhamnose inducible promoter. L-rhamnose and L-mannose were added to bacteria culture with final concentration of 0, 0.1, 0.4, 0.7 mg/mL. The fluorescence intensity of EYFP and OD600 was measured immediately and synchronously after 10 h induction. Experimental results are shown in Fig. 4.
Fig. 4 Characterization of the eyfp expression and bacterial growth in different inducer concentrations (a: L-rhamnose, b: L-mannose).
From the experiment results, we can obviously draw the conclusion that the L-rhamnose induction effect is better than L-mannose. The normalized RFU rises with increasing L-rhamnose concentration. In contrast, the normalized RFU shows a declined tendency when the concentration of L-mannose rises to a certain value and its OD600 keeps decreasing as the concentration increased. We guess that we use similar L-mannose induction concentration as the reference, but the gene circuit and strain are totally different from its, which may be the reason for different results. What is more, we believe that under our experiment circumstance, it will produce bacteria toxicity when L-mannose induction concentration reach a certain value. Apparently, L-rhamnose is the better inducer for this improved system.
Reference
1. C. L. Kelly et al., Synthetic Chemical Inducers and Genetic Decoupling Enable Orthogonal Control of the rhaBAD Promoter. ACS Synth. Biol. 5, 1136-1145 (2016). Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 440
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 172
- 1000COMPATIBLE WITH RFC[1000]
| None |