Part:BBa_K4011002
Ma-Mut
Ma-Mut is a mutated protein tag derived from the protein secretion tag (Ma) of mating type alpha Saccharomyces cerevisiae. In yeast cells, proteins with the Mα tag will be secreted out of the cell. This year, we will use Mα-Mut to enable secretion of proteins fused with cellulose binding domain 3 (CBM3) from yeast. CBM3 BBa_K4011000 is an artificial protein derived from Ruminiclostridium thermocellum (Protein Data Bank (PDB) accession: 1NBC) and will bind to cellulose fibers. This will allow for modification of our bacterial cellulose membrane produced by a co-culture of Komagataeibacter and yeast. We will use Ma_Mut to construct composite parts Mα-sfGFP-CBM3 BBa_K4011010 and Mα-CBM3-NT2RepCT-CBM3 BBa_K4011011.
Other teams can utilize our Ma sequence for S. cerevisiae protein secretion.
Source
Ma comes from Saccharomyces cerevisiae.
Usage and Biology
The alpha-factor preproleader sequence is responsible for protein secretion in mating type alpha Saccharomyces cerevisiae. Mα_Mut is derived from the preproleader sequence and first characterized by Aza et al in 2021, who mutated the sequence to increase protein secretion efficiency.
Natural Mα contains 89 amino acids with three functional regions: a pre-region (19 amino acids), pro-region (64 amino acids), and a spacer (6 amino acids). During S. cerevisiae modification, the preproleader will translocate the protein across the Endoplasmic Reticulum (ER), and the pre-region will be cleaved. Then, the pro-region will direct the protein to the Golgi Apparutus for final modifications before release into media. (Aza et al., 2021)
CBM3s allow for fused proteins to be bound to cellulose.
Characterization
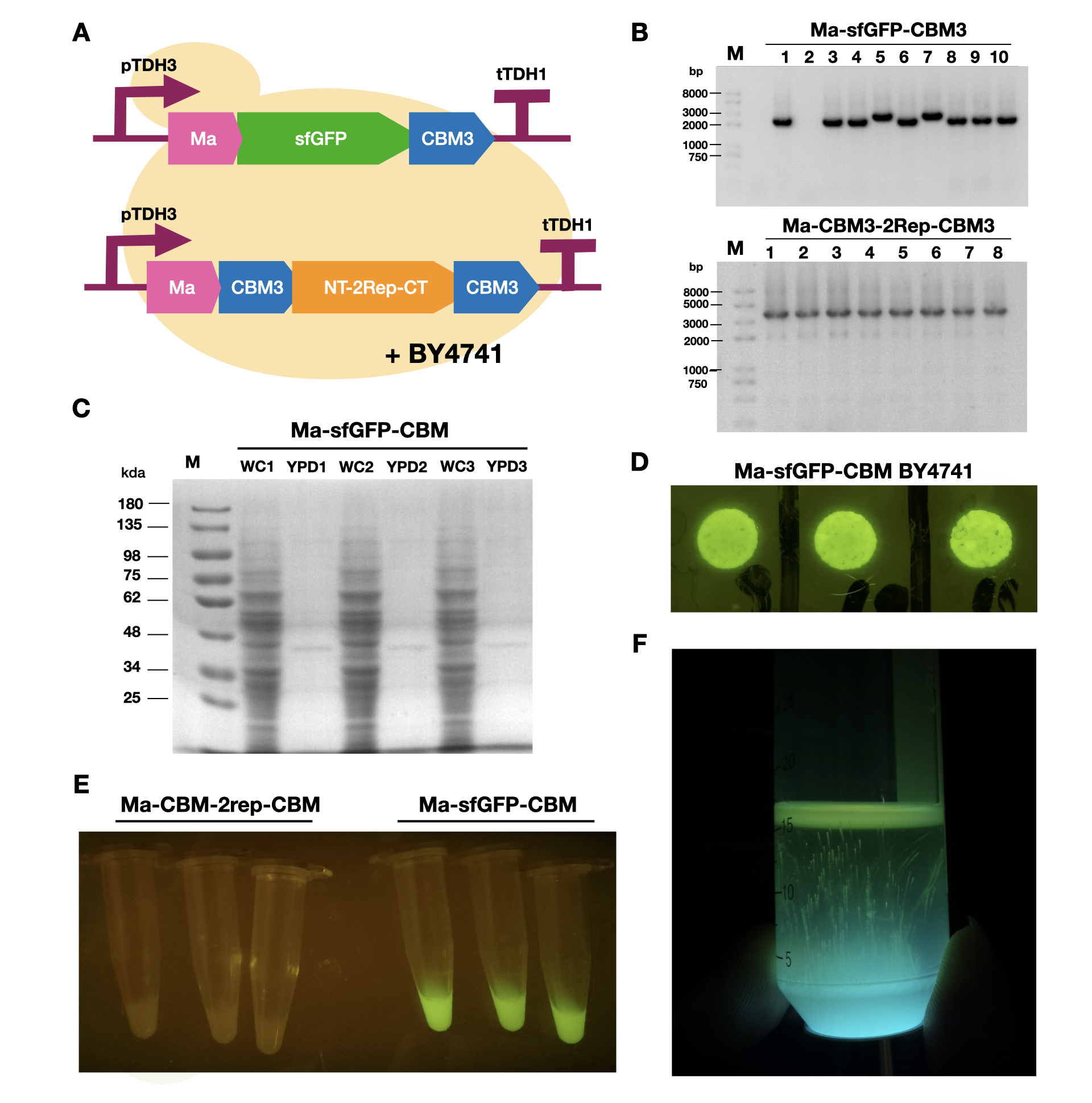
To enable secretion of proteins in yeast, we attached a short signal peptide called maturation factor alpha (Ma) to our protein. Aza et al have already characterized Mα and mutated it to become more efficient at protein secretion. For our project, we selected Ma A9D; A20T; Q32H; F48S; G62D (referred to as Ma_Mut) as it had one of the highest secretion rates. We constructed two plasmids, Ma-sfGFP-CBM3 and Ma-CBM3-2Rep-CBM3 (Fig. 1B), and transformed both plasmids into yeast (Fig. 1A).
On YPD plates, secretion of Ma-sfGFP-CBM3 can be seen by the green halo surrounding each individual colony (Fig. 1D). After culturing Ma-sfGFP-CBM3 and Ma-CBM3-2Rep-CBM3 yeast in liquid YPD (Fig. 1E), we performed SDS-PAGE analysis on both the whole cell and growth media (Fig. 1C). We discovered that only a small portion of the expressed proteins were found in the media for Ma-sfGFP-CBM3, and an unobservable amount of secreted protein was found in the Ma-CBM3-2Rep-CBM3 media. This meant that most likely, only a small amount of CBM3-2Rep-CBM3 will be secreted, not enough to significantly alter the characteristics of the resulting BCM. As a proof of concept, we cultured SCOBY using Ma-sfGFP-CBM3 (Fig. 1F). In the future, we hope to increase the expression of secretion rate of Ma-sfGFP-CBM3 and Ma-CBM3-2Rep-CBM3 to achieve in situ modification of BCM (Mohammadi et al, 2019).
Design Considerations
1. The following mutations are added in Mα_Mut: A9D, A20T, Q32H, F48S, and G62R.
2. The last eight amino acids is a linker with the sequence EAEAEFGS.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
References
Aza, P., Molpeceres, G., de Salas, F. et al. Design of an improved universal signal peptide based on the α-factor mating secretion signal for enzyme production in yeast. Cell. Mol. Life Sci. 78, 3691–3707 (2021). https://doi.org/10.1007/s00018-021-03793-y
| None |
