Part:BBa_K3904228
Surface-layer protein A promoter (p-slpA)
To assure continuous and efficient naringenin production, we had to guarantee the proper expression of naringenin synthesis enzymes in our probiotic strains. To reach the maximum efficiency of the natural synthesis pathway, we decided to manipulate the expression rates of these proteins by finding the promoters of optimal strength for the expression of each of these enzymes.
Introduction
Vilnius-Lithuania iGEM 2021 project AmeByelooks at amebiasis holistically and comprehensively, therefore target E. histolytica from several angles: prevention and diagnostics. Our team's preventive solution consists of probiotics engineered to produce naringenin - an antiprotozoal compound. Two strains of genetically modified microorganisms were chosen as the main chassis - world-renowned Lactobacillus casei BL23 (Lactobacillus paracasei) and Escherichia coli Nissle 1917. Furthermore, the team made specific gene deletions to enhance naringenin production and adapted a novel toxin-antitoxin system to prevent GMO spreads into the environment. The diagnostic part includes a rapid, point of care, user-friendly diagnostic test identifying extraintestinal amebiasis. The main components of this test are aptamers specific to the E. histolytica secreted proteins. These single-stranded DNA sequences fold into tertiary structures for particular fit with target proteins.
Contents
Usage and Biology
P-slpA features
The Lactobacillus acidophilus ATCC 4356 slpA promoter demonstrates the highest levels of transcription in all Lactobacillus strains examined in the study conducted by A. McCracken and colleagues. Combined with data from other researchers indicating that the slpA promoter is highly efficient in the natural host and in L. casei ATCC 393, this suggests that the slpA promoter sequence harbours motifs that interact effectively with RNA polymerase of several lactobacilli. This has striking implications for the development of heterologous gene-expression cassettes in lactobacilli by offering a powerful tool for achieving high levels of foreign protein production [1].
Overcoming metabolic pathway limitations
We engineered a metabolic pathway for naringenin production in E. coli Nissle 1917 by using mathematical modeling to pick promoters. SlpA promoter was used to produce the enzyme responsible for the bottleneck reaction as this sequence was the strongest one of the ones that were evaluated.
Part characterization
SlpA strength evaluation by sfGFP expression
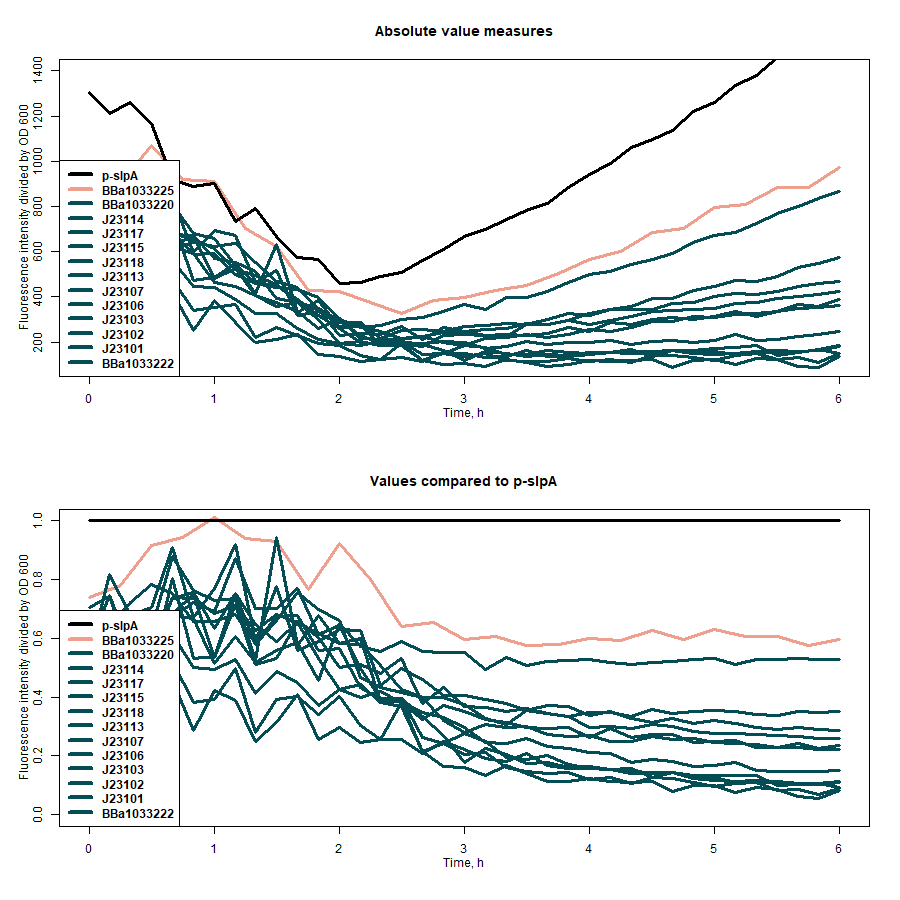
Promoter strength was estimated by measuring how intensively the E. coli Nissle 1917 transformants can produce super folder GFP (sfGFP) under the promoter of interest. Fluorescence intensity was evaluated by dividing the intensiveness of the signal by the OD600 of the medium during the course of 7 hours. The greater ratio would indicate the stronger promoter, in this case - slpA (Fig. 1).
Figure 1: first graph - comparison of the strength of all target promoters; second graph - the strength of all target promoters compared to p-slpA.
The promoter strength measuring data was compared between all the promoters of our interest taking the strongest splA promoter as a reference point (Table 1).
Table 1: summarized promoter evaluation results
| Promoter | Absolute value of fluorescence/OD600 at the midpoint of growth | Promoter strength in comparison to p-slpA |
|---|---|---|
| BBa_1033225 | 383.99 | 0.76 |
| BBa_1033222 | 227.79 | 0.45 |
| BBa_1033222 | 295.25 | 0.58 |
| P-slpA | 508.22 | 1.00 |
| J23118 | 176.43 | 0.35 |
| J23117 | 155.74 | 0.31 |
| J23115 | 135.46 | 0.27 |
| J23114 | 175.06 | 0.34 |
| J23113 | 180.56 | 0.36 |
| J23107 | 253.16 | 0.50 |
| J23106 | 237.59 | 0.47 |
| J23103 | 178.62 | 0.35 |
| J23102 | 187.82 | 0.37 |
| J23101 | 226.14 | 0.44 |
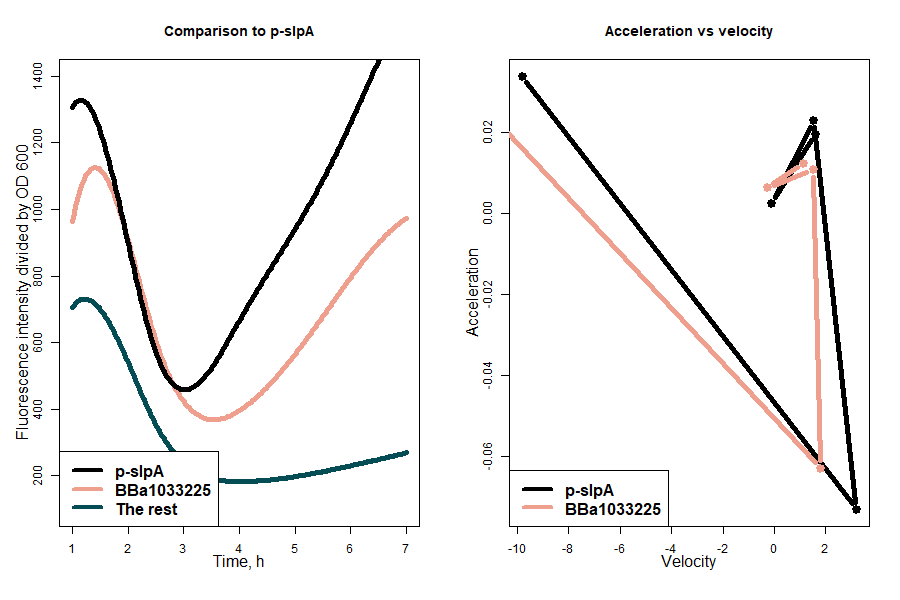
Two sequences demonstrating the greatest sfGFP expression rates, the strongest one being p-slpA, were compared to generalized expression intensity of the remaining weaker promoters. Seeking better insight, the expression acceleration and velocity under slpA and BBa_1033225 promoters were also evaluated. The results showed that besides being two strongest indicated promoters, these sequences also share similar expression dynamics (Figure 2).
Figure 2: first graph - comparison of promoter strength by indicating the curves of the two strongest promoters and the generalized curve of the remaining weaker promoters; second graph - comparison of acceleration versus velocity of two strongest promoters.
Expression under slpA promoter after inserting the target construct into E. coli Nissle 1917 genome
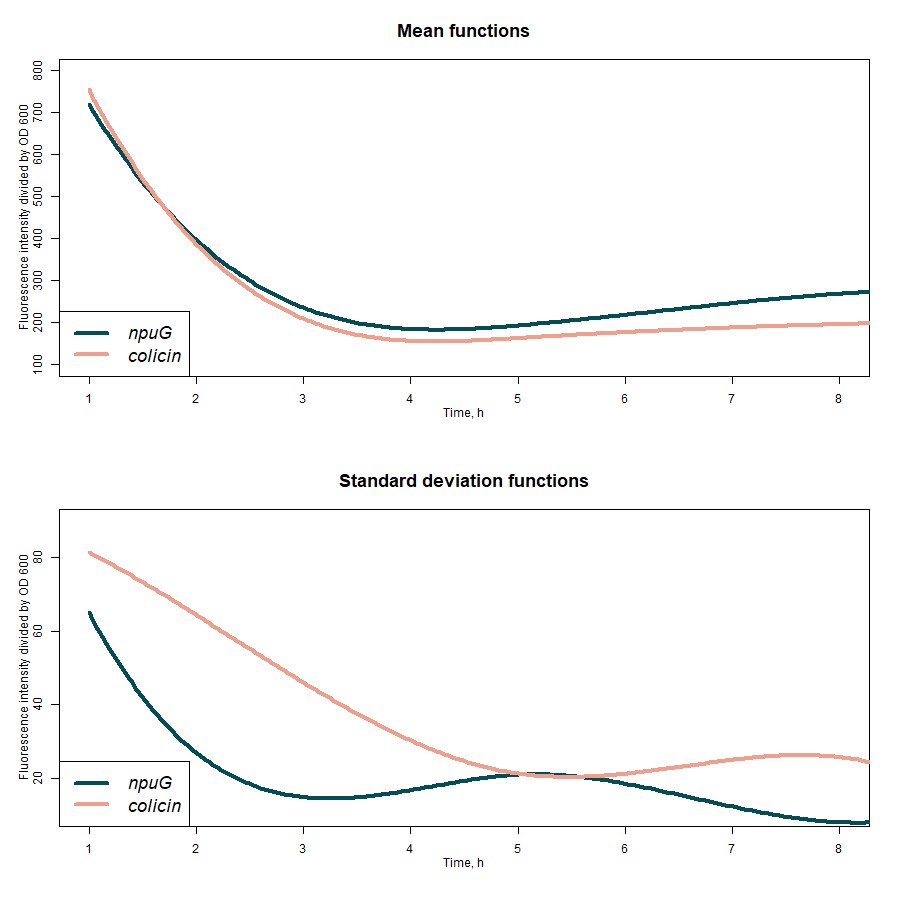
Particularly in our project, we needed to insert the metabolic pathway for naringenin production into the genomes of our target probiotic strains using the CRISPR-Cas9 system. We have chosen two genomic sites for genomic insertions, which would do not harm or negatively affect our probiotic bacteria growth or overall performance - a putative colicin-encoding and nupG genes. We have inserted sfGFP encoding gene under the control of constitutive slpA promoter into these sequences. sfGFP fluorescence was significantly higher in samples with insertion in nupG, however, this case also demonstrated significantly higher fluorescence fluctuations in comparison with putative colicin samples (Figure 3).
Figure 3: sfGFP expression rates under slpA promoter from its constructs insertion in nupG and putative colicin genes.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
References
- McCracken, A., Turner, M. S., Giffard, P., Hafner, L. M., & Timms, P. (2000). Analysis of promoter sequences from Lactobacillus and Lactococcus and their activity in several Lactobacillus species. Archives of microbiology, 173(5), 383-389.
| None |