Part:BBa_K3757009
35S sGFP (S65T)
Green fluorescent protein variant sGFP (S65T) (BBa_K3669012) gene, regulated by CaMV 35S promoter (BBa_K788000) and 35S terminator (BBa_K1159307). This part can be used to constitutively express GFP in Nicotiana benthamiana.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 1575
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 66
Usage and Biology
This part can be used for the expression of the fluorescent protein sGFP (S65T) in plants. It inherits the following basic parts:
- CaMV 35S promoter (BBa_K788000)
- sGFP (S65T) (BBa_K3669012)
- 35S terminator (BBa_K1159307)
The iGEM team Tuebingen 2021 used this part as an expression control in plants. In our project, we wanted to express stabilized antimicrobial peptides (AMPs) in Nicotiana benthamiana (N. benthamiana). Therefore, we co-expressed sequences encoding a precursor peptide (BBa_K3757001) containing the stabilized AMPs with a cyclizing asparaginyl endopeptidase CtAEP1 (BBa_K3757000), which catalyzes cyclization of the expressed peptides1. To allow a fast observation of the transfection and expression progress in the plants, we also co-expressed sGFP (S65T), hereafter called sGFP. This could then be easily observed by fluorescence microscopy.
All three genes, encoding the peptide precursor, CtAEP1 and sGFP, were cloned into a binary vector for agroinfiltration, leading to our 3in1 gene cassette (BBa_K3757011) for co-expression of three genes. sGFP was thereby regulated by the constitutive eukaryotic promoter CaMV 35S (BBa_K788000) and a 35S terminator polyadenylation signal (BBa_K1159307), forming the composite part BBa_K3757009.
Expression
After successful cloning, the purified vectors were transformed into Agrobacterium tumefaciens for the following agroinfiltration of tobacco plants. After growing the plants for 4 days, the recombinant protein expression of our control green fluorescent protein (sGFP) was confirmed by fluorescence microscopy and the leaves were harvested for protein extraction and purification.
Nicotiana benthamiana leaves were infiltrated with A. tumefaciens containing the respective vectors and a p19 suppressor of gene-silencing construct. Co-transfection of p19 is essential, as p19 prevents that the addition of the vector leads to RNA-induced gene silencing2. We infiltrated the tobacco leaves by syringe infiltration for transient protein expression. As a negative control, leaves were infiltrated with Agrobacteria containing only the p19 construct.
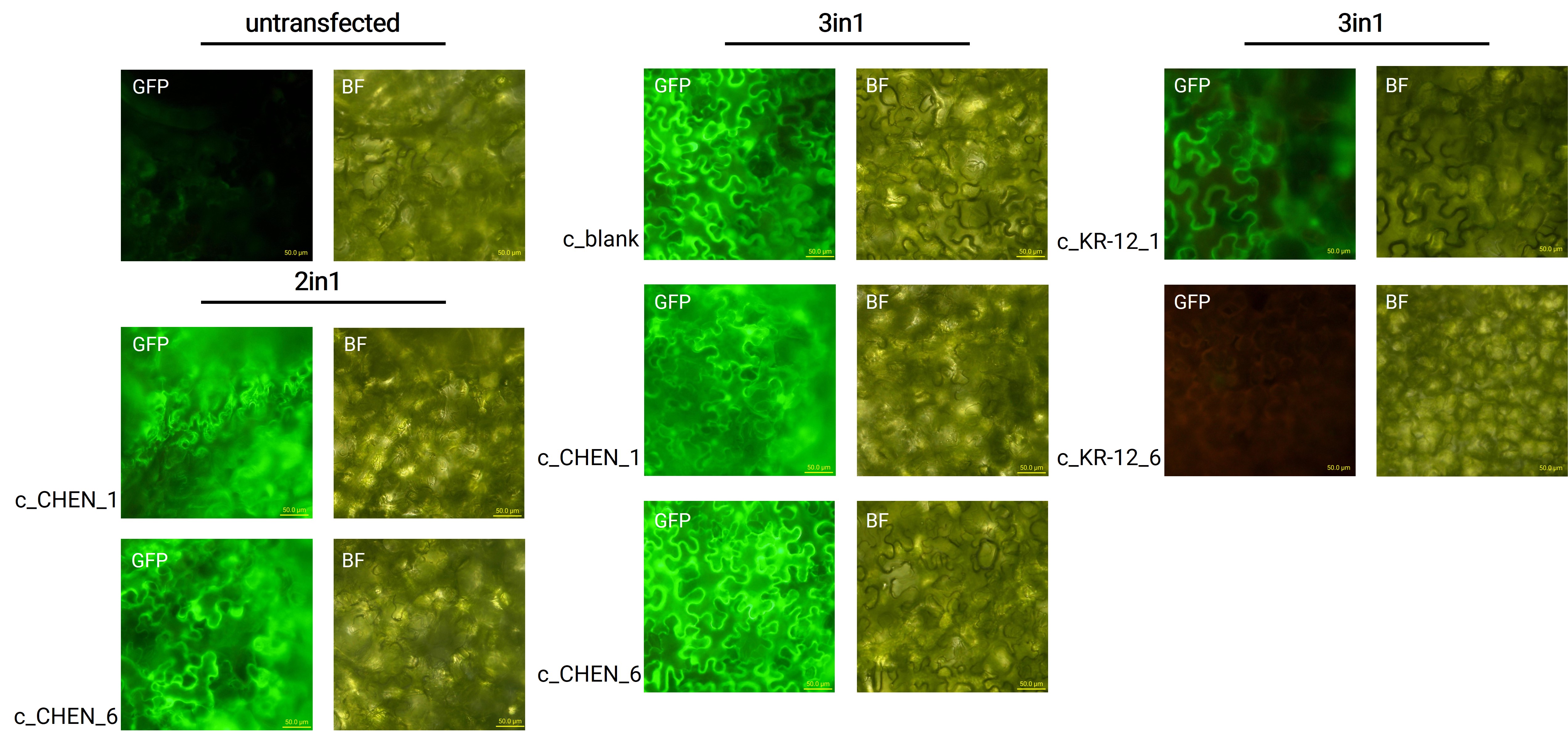
After growing the plants for 4 days, the expression of our expression control protein sGFP was confirmed by fluorescence microscopy. In comparison to the negative control, leaves transfected with our final vectors showed clear green fluorescence for all except the c_KR-12_6 construct (figure 1). As a negative control, non-infiltrated leaves or leaves infiltrated with p19 only were checked for green fluorescence. As shown in figure 1, this negative control showed no significant level of green fluorescence, confirming that the green fluorescence we detected for other vectors can only result from successful expression of one of our final vectors.
Conclusion
We were able to demonstrate the successful expression of sGFP from our 3in1 gene cassette (BBa_K3757011) in transiently transfected N. benthamiana (figure 1). Even though later on, the yield of our expressed peptides was quite low, the sGFP fluorescence observed was very bright and clearly detectable as a positive expression control signal.
References
1Poon, S., Harris, K. S., Jackson, M. A., McCorkelle, O. C., Gilding, E. K., Durek, T., van der Weerden, N. L., Craik, D. J., & Anderson, M. A. (2018). Co-expression of a cyclizing asparaginyl endopeptidase enables efficient production of cyclic peptides in planta. Journal of Experimental Botany, 69(3), 633–641. https://doi.org/10.1093/jxb/erx422
2Voinnet, O., Rivas, S., Mestre, P., & Baulcombe, D. (2003). An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. The Plant Journal: For Cell and Molecular Biology, 33, 949–956. https://doi.org/10.1046/j.1365-313X.2003.01676.x
| None |