Part:BBa_K3743007
L7Ae (dCas13) linked by Gly Ser Linker
Part Description
Cas13 proteins recently identified RNA-guided and RNA-targeting RNase protein family which are single-component programmable RNases with functions in RNA processing and programmed RNA cleavage. which is linked by Gly Ser Linker with "L7AE" which is a member of a protein's family that binds K-turns in RNA to stabilize the tightly kinked conformation. They are very popular and are important in the assembly of RNA–protein complexes central to translation, splicing and site-specific RNA modification. Cas13 binds to mRNA from the cancerous environment and consumes L7Ae, disinhibiting the circuit and enabling more copies to be created.
Usage
It is a deactivated Cas13 used in conjugation with L7Ae protein (that has an inhibitory effect on the transcription by binding to its kink-turn) by Gly Ser linker. The system simply recognizes and binds to mRNA in the cancerous environment which leads to consumption of L7Ae protein. Therefore, not binding to kink-turn to inhibit the inhibitory effect on the transcription which finally leads to stimulation of the transcription in order to increase the yield of the vaccine. That is why, it is a cell specific design by binding to mRNA of PD-L1 which has an immune evasion role in the cancerous environment especially TLCs.
Literature Characterization
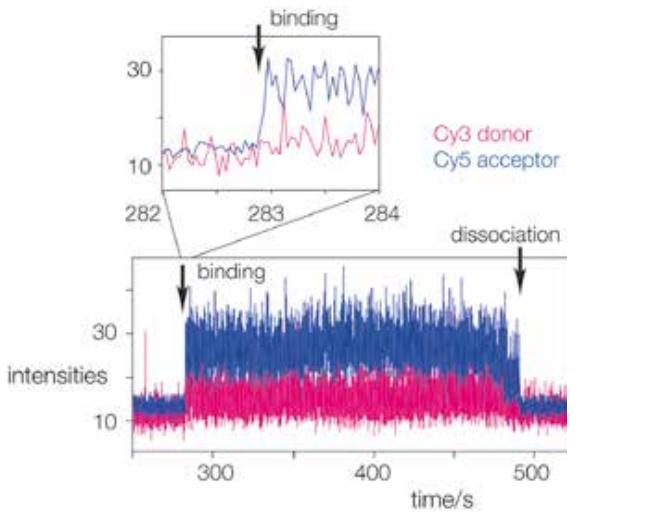
Real time single-molecule FRET observation of RNA folding when bound to the L7Ae protein is performed. The binding of terminally donor-acceptor fluorescently labelled RNA to the immobilised L7Ae protein can be seen. When the RNA binds to the L7Ae, it folds, increasing FRET efficiency and increasing the intensity of the Cy5 acceptor (blue trace) as shown in figure(1). The inset illustrates an extension of the region where the RNA binds to the protein, resulting in an immediate rise in FRET efficiency, indicating a conformational capture mechanism.(1)
Characterization Of Mutational Landscape
After performing mutagenesis prediction of mutational landscape of L7Ae and tested the effect of these mutations on the evolutionary fitness of the protein after generating multiple sequence alignment of the protein sequence and predict mutational landscapes. As shown in the chart, the (G108S) mutation showed the highest score compared to other mutations. On the contrary, we can see that the (G108K) contributed to the lowest evolutionary fitness to L7Ae. As shown in Figure (2). Which was used to also charectrize and improve part BBa_K2100068
Also After performing mutagenesis prediction of mutational landscape of dcas13 and tested the effect of these mutations on the evolutionary fitness of the protein after generating multiple sequence alignment of the protein sequence and predict mutational landscapes. As shown in the chart, the (A472R) mutation showed the highest score compared to other mutations. On the contrary, we can see that the (A477K) contributed to the lowest evolutionary fitness to dcas13. As shown in Figure (3)
Characterization by Mathematical Modelling
In order to simulate the dynamics of the used riboswitches which acts as an essential safety switches in our vaccine design, Mathematical modeling is performed using ordinary differential equations (ODEs) and fitted parameters.
As shown in Figure(4), P2 which represents the binding state shown inhibition throughout the time which conclude inhibition of the circuit. On the other hand, P1 which represents removing the inhibitory effect of riboswitches showed increased transcription of the circuit which reach the steady state after about 200 seconds. Which was used to also charectrize and improve part BBa_K2100068
References
1.Lilley, D. M. (2019). The L7Ae proteins mediate a widespread and highly functional protein–RNA interaction. The Biochemist, 41(2), 40-44.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 1117
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 3396
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
| None |