Part:BBa_K3584006
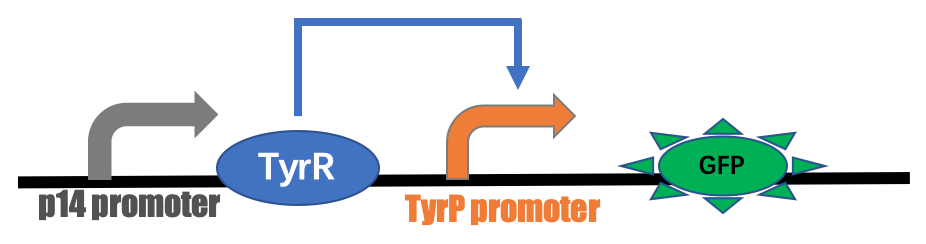
p14 pro-rbs-tyrR-rbs-tyrP-GFP
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 798
Illegal BglII site found at 1140 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 604
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 2485
In Escherichia coli, the TyrR protein can both repress and activate the transcription operons required for the biosynthesis and uptake of aromatic amino acids (tyrosine, phenylalanine, and tryptophan). For example, the promoter of the tyrP gene (encodes a tyrosine-specific transporter) is activated by the TyrR dimer in the presence of tyrosine.
Contribution
BBa_K3584006 is composite part containing the coding sequences of TyrR gene and green fluorescent protein (GFP) which are controlled by constitutive promoter p14 and tyrP promoter, respectively. TyrR is transcriptional regulatory protein that is involved in transcriptional regulation of aromatic amino acid biosynthesis and transport. The activity of the TyrR protein is modulated by the binding of one or more of the aromatic amino acids (phenylalanine, tyrosine and tryptophan). These amino acids act as co-effectors which bind to the TyrR protein to form an active regulatory protein. TyrR causes positive effects on the tyrP promoter. Our team aimed to alleviate the harm from gut-derived toxins in patients of chronic kidney disease by using synthetic live bacteria gut metabolize toxins, which E. coli was engineered to express polyphenol oxidase (PPO) proteins. Moreover, PPO protein degrades p-cresol (precursor of p-cresyl sulfate), making it impossible to synthesize toxin which is harmful to renal and heart function. And the tyrosine operon is used as a biological switch, which is induced by dietary amino acid, to produce toxin-degrading enzyme in appropriate time. As the beginning of the project, the part BBa_K3584006 was constructed as a GFP reporting system to verify the function of the tyrosine induced promoter.
Engineering Success
GFP reporting system to sense the aromatic amino acids The part BBa_K3584006 was constructed on the pSU2718-p15A vector. The constructed plasmids were transformed into Escherichia coli Nissle 1917(EcN) receptive state of intestinal probiotics. The recombinant strains were cultured with addition of different concentrations of tyrosine. As shown in Figure 1, the fluorescence intensity of E. coli was obviously seen under UV light.
Figure 1. Fluorescence intensity of E. coli containing part BBa_K3584006 under UV light.
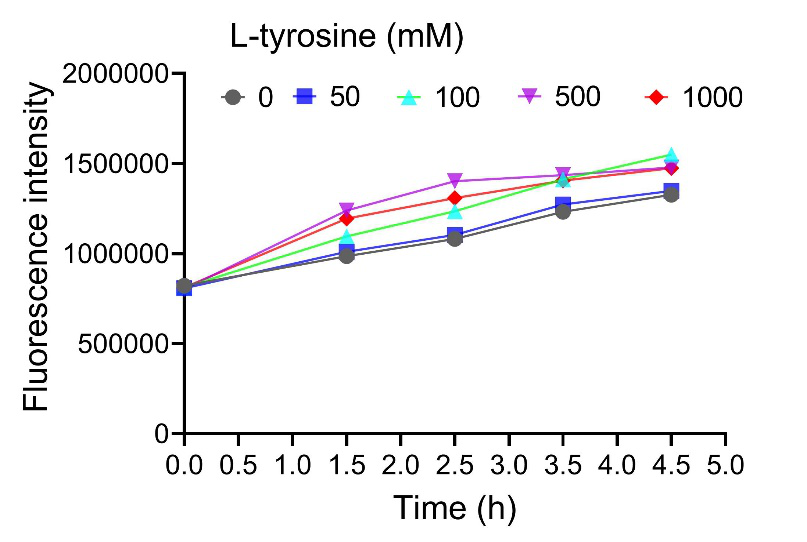
The fluorescence intensity increased with the increase of tyrosine concentration, indicating that the induction system responded well to the tyrosine concentration in E. coli Nissle 1917. When we increased the concentration of tyrosine to 500 μM, the fluorescence intensity was further enhanced, but when the concentration reached 1000 μM, the fluorescence intensity of the strain decreased, so the high concentration of amino acids may have an inhibitory effect on the strain (Figure 2).
Figure 2. Fluorescence intensity of E. coli Nissle 1917 with different tyrosine concentration.
Enzymatic activity of the PPO
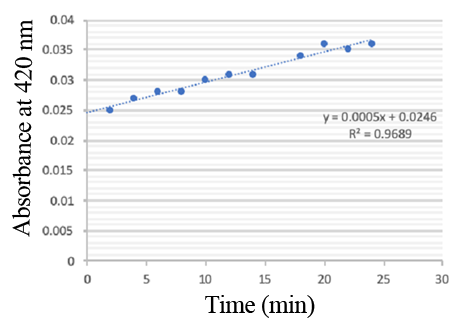
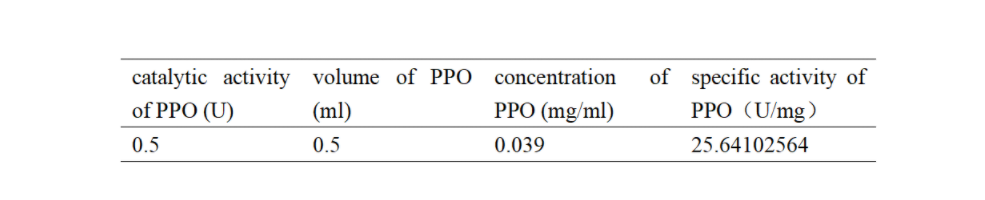
PPO activity was determined using an analogue of p-cresol (catechol) as a substrate. In proper reaction mixture, PPO and 10 mM catechol was incubated at 50 ℃, pH 7.0 for 3 min, and the change in absorbance at 420 nm was measured spectrophotometrically. One unit of enzyme (PPO) activity was defined as the amount of enzyme that increased absorbance of 0.001 per minute. We found that as the extension of reaction time, reaction liquid of absorbance at 420 nm increased linear (Figure 2), we obtained by catalytic activity of PPO enzyme fluid, and divided by the corresponding protein concentration, the specific activity of PPO (Table 1).
Figure 3. PPO activity was determined using catechol as a substrate. The reaction mixture contained PPO protein and 10 mM catechol which was incubated at 50 ℃, pH 7.0 for 3 min, and the change in absorbance at 420 nm was recorded.
Table 1. Specific activity of recombinant PPO
| None |