Part:BBa_K3419003
Contents
Tricosanthin with Strong Promoter and TorA T
Usage and Biology
Note: All work on this part was done virtually through literature research due to COVID-19 restrictions.
Trichosanthin is a protein derived from Trichosanthes kirilowii, also known as the Chinese cucumber. It has been used in Eastern traditional medicine as an abortifacient and also functions as an antitumor agent due to its inactivation effect on eukaryotic ribosomes [1]. Trichosanthin is used in our project as an anti-cancer therapeutic protein that selectively kills tumor cells while leaving our engineered prokaryotic E. coli unaffected. We were able to confirm that E. coli would be able to produce this therapeutic based on a literature search [2]. Thus, our bacteria would perform their intended function in our genetic circuit.
Mechanism
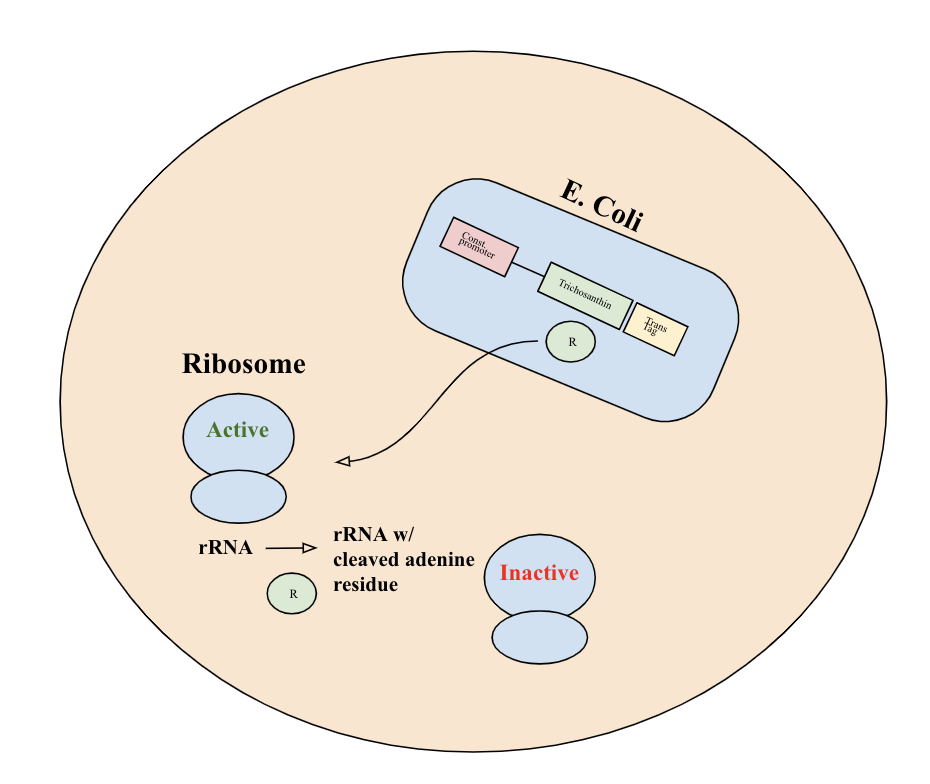
Trichosanthin is produced constitutively by the engineered bacteria upon entry, and is exported into the cytoplasm of the tumor cells. Upon release into the cell, trichosanthin exhibits RNA N-glycosidase activity and depurinates the 28S rRNA of the 60S eukaryotic ribosomal subunit. Ribosome inhibition is achieved by interaction of trichosanthin with the ribosomal stalk protein P2 C-terminal tail. Binding to the C-terminal domain of ribosomal P protein achieves irreversible inactivation of eukaryotic ribosome activity and inhibition of tumor cell function [3]. Trichosanthin does not affect prokaryotic ribosomes, which allows E.coli to remain functional and act as a therapeutic delivery system. Since trichosanthin acts by inhibiting protein synthesis, its cytotoxic effects will be most deleterious to fast-growing cancer cells [4]. Additionally, our addition of a translocase tag allows for more efficient export of trichosanthin from the bacteria into the cytoplasm of the tumor cell [5].
Part Details
This BioBrick part consists of the trichosanthin coding gene BBa_K3419000. It is driven by a constitutive promoter (BBa_J23100), has a ribosome binding site (BBa_J61100), and a terminator (BBa_B0015). It also includes a translocase tag (BBa_K2960001), which facilitates the export of the protein from E. coli into the tumor cell.
Modeling
In MATLAB, we modeled the output of the Trichosanthin protein using the following differential equation, and we solved it using MATLAB’s ode45 function. The equation represents the production of Trichosanthin protein. The first term represents the basal expression under the constitutive promoter (BBa_J23100), while the second represents protein degradation.
The MATLAB scripts corresponding to the Trichosanthin modeling can be downloaded here:
File:ModelingCodeCornelliGEM2020.zip
| Variable | Parameter | Value | Source |
|---|---|---|---|
| [Trich] | Concentration of Trichosanthin | -- | -- |
| basT | Basal transcription rate | 0.387 μM/sec | [3] |
| dC | degradation rate of Trichosanthin | 0.000385 sec-1 | [4] |
Figure 2 shows that the E. coli are able to produce a sufficient concentration of trichosanthin to kill the cancer cells given that about one hundred bacteria will colonize in one cell according to our growth modeling [8,9]. Each bacteria causes a concentration increase of 268 nM, which implicates a total of 26.8 μM of trichosanthin in the cancer cell, which is significantly higher than the IC50 of 1.57 μM. A breast cancer study in mice showed that trichosanthin administered intravenously at 0.185 μM significantly slowed cancer proliferation [2]. Since Trichotherapy applies a much higher concentration than this effective chemotherapy concentration as well as the IC50, it would likely destroy cancer cells and significantly reduce tumor size. We do not expect the high concentration to be harmful as our built-in kill switch is an added safety defense to limit bacterial spread outside of the tumor.
References:
[1] Li, M. X., Yeung, H. W., Pan, L. P., & Chan, S. I. (1991). Trichosanthin, a potent HIV-1 inhibitor, can cleave supercoiled DNA in vitro. Nucleic acids research, 19(22), 6309–6312. https://doi.org/10.1093/nar/19.22.6309
[2] Zhu, R., Ng, T., Yeung, H., & Shaw, P. (2009). High level synthesis of biologically active recombinant trichosanthin in Escherichia coli. International Journal of Peptide and Protein Research, 39(1), 77-81. doi:10.1111/j.1399-3011.1992.tb01558.x
[3] Shi, W., Wong, K., & Shaw, P. (2018). Structural and Functional Investigation and Pharmacological Mechanism of Trichosanthin, a Type 1 Ribosome-Inactivating Protein. Toxins, 10(8), 335. doi:10.3390/toxins10080335
[4] Li, J., Li, H., Zhang, Z., Wang, N., & Zhang, Y. (2016). The anti-cancerous activity of recombinant trichosanthin on prostate cancer cell PC3. Biological research, 49(1), 21.
[5] Fang, E. F., Zhang, C. Z., Zhang, L., Wong, J. H., Chan, Y. S., Pan, W. L., . . . Ng, T. B. (2012). Trichosanthin Inhibits Breast Cancer Cell Proliferation in Both Cell Lines and Nude Mice by Promotion of Apoptosis. PLoS ONE, 7(9). doi:10.1371/journal.pone.0041592
[6] Yildirim, Necmettin, and Michael C Mackey. “Feedback regulation in the lactose operon: a mathematical modeling study and comparison with experimental data.” Biophysical journal vol. 84,5 (2003): 2841-51. doi:10.1016/S0006-3495(03)70013-7
[7] Rust, Aleksander et al. “The Use of Plant-Derived Ribosome Inactivating Proteins in Immunotoxin Development: Past, Present and Future Generations.” Toxins vol. 9,11 344. 27 Oct. 2017, doi:10.3390/toxins9110344
[8] Duong, M.T., Qin, Y., You, S. et al. Bacteria-cancer interactions: bacteria-based cancer therapy. Exp Mol Med 51, 1–15 (2019). https://doi.org/10.1038/s12276-019-0297-0
[9] Toley, B. J., & Forbes, N. S. (2011). Motility is critical for effective distribution and accumulation of bacteria in tumor tissue. Integrative Biology, 4(2), 165-176. doi:10.1039/c2ib00091a
| None |