Part:BBa_K3264002
CT(C-terminal domain of spidroin)
Minispidroin contains an CT from A.ventricosus MiSp E. australis(minor ambulate spidorins). The C-terminal domain is a non-repetitive domain that is highly soluble and pH sensible. In pH=7 solution, it’s a folded dimeric domain, however, as pH decreases, it starts to unfold. The unfolding process was hyposized to generate the formation amyloid-like fibrils that enables transition of the Rep (the repetitive region) into beta-sheet corroboration. NT-2Rep-CT is the recombinant spider silk protein (spidroin) that can spin into artificial spider silk fiber. This part is in the part collection where we provide improved recombinant chromoproteins and spidroins based on the functions of different regions of naturally occurring spidroin. By carrying out different mixing of the proteins of different combinations, we can produce artificially spun silk of different colors and properties.
The part collection includes: Parts that are for formation of spidroins for silk spining. BBa_K3264000 BBa_K3264001 BBa_K3264002 BBa_K3264003 BBa_K3264004 BBa_K3264005 BBa_K3264007 BBa_K3264009 BBa_K3264018. BBa_K3264019. Parts that for combining with the spidroins to form color silks BBa_K3264012. BBa_K3264013. BBa_K3264014. BBa_K3264015. BBa_K3264016. BBa_K3264017. Part for adding more extensive repetitive region to the spidroin protein BBa_K3264011.
Our part collection can help and inspire future teams to further design recombinant spider silks of more variety and give silks diversified color and property to fulfill the collection.
Reference
[1] Andersson, Marlene, et al. “Biomimetic Spinning of Artificial Spider Silk from a Chimeric Minispidroin.” /Nature Chemical Biology/, vol. 13, no. 3, Sept. 2017, pp. 262–264., doi:10.1038/nchembio.2269.
Usage and Biology
Spider silk serves as a new material with superior properties that can be applied in medication, cloth, and aerospace fields. However, spider breeding is not applicable due to spider's fierce behavior. The current approach is to produce recombinant spidroins (silk proteins) from other chassis and spin them into silk.
NT-2Rep-CT is the common architecture in two main types of spider silk proteins: major ambulate spidroins(MaSps) and minor ampullate spidorins (MiSps). NT is a non-repetitive N-terminal domain, as well as CT, the C-terminal domain. In between them is an extensive repetitive region. In the storage of spider silk protein, NT is a monomeric and highly soluble region in neutral pH, and it provides the solubility of the entire spidorin. NT can form stable dimers when pH decreases in the spinning duct. On the other side during the decrease of pH, the CT gets disrupted and unfold to turn in the beta-sheet amyloid-like fibrils. It is hypothesized that structural change of CT precipitate the change of the repetitive region to beta-sheet conformation. Based on the part NT-2Rep-CT, development was carried out by our group to enable a step closer the recently unattainable size of nature spidroin(556 kDa) that contains192 repeat motifs of the Nephila clavipes dragline spidroin. More details please go to NT4RepCT. (Andersson, Marlene, et al. 2017)
Design Considerations
We found the amino acid sequence of NT domain, CT domain and extensive region Rep. Codon optimization was carried out by us to code the nucleotide sequence of NT-2Rep-CT according to the E.coli codon bias. The construct was cloned into a pT7 plasmid and transformed into BL21 (DE3) E. coli. The construction includes:
1. a 6× His tag (MGHHHHHHM) is added to enable us carrying out Ni-NTA protein purification
2. an N-terminal domain based on the E. australis MaSp1 sequence (SHTTPWTNPGLAENFMNSFMQGLSSMPGFTASQLDDM STIAQSMVQSIQSLAAQGRTSPNKLQALNMAFASSMAEIAASEEGGG SLSTKTSSIASAMSNAFLQTTGVVNQPFINEITQLVSMFAQAGMNDVSA; EMBL accession number AM259067)
3. a repetitive part consisting of two polyalanine and glycine-rich repeat regions from MaSp1 of E. australis (GNSGRGQGGYGQGSGGNAAAAAAAAAAAAAAAGQGGQGGYGR QSQGAGSAAAAAAAAAAAAAAGSGQGGYGGQGQGGYGQSGNS; EMBL accession number AJ973155)
4. a C-terminal domain based on the A. ventricosus MiSp sequence (VTSGGYGYGTSAAAGAGVAAGSYAGAVN RLSSAEAASRVSSNIAAIASGGASALPSVISNIYSGVVA SGVSSNEALI QALL ELLSALVHVLSSASIGNVSSVGVDSTLNVVQ DSVGQYVG; GenBank accession number JX513956).
Also, the linker between the NT/CT and the 2Rep(repetitive region) is GNS
Characterization
We aimed to use a synthetic biology approach to combine standardized DNA part NT-2Rep-CT, and transform constructs to metabolically engineered E. coli for bioproduction.
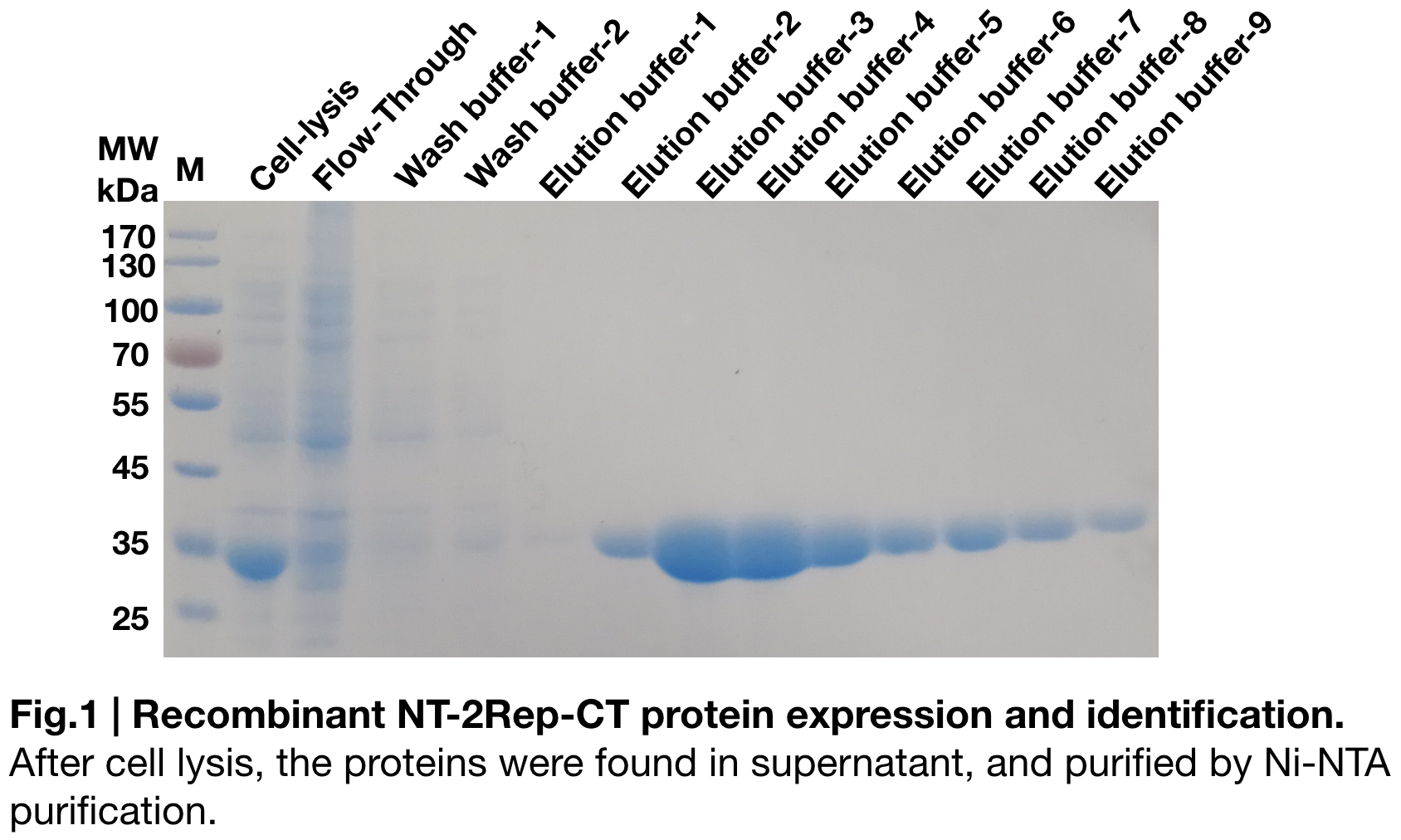
Purification and SDS PAGE
In order to detect whether protein expression was induced by adding isopropylthiogalactoside (final concentration 0.3mM), we used SDS-page(12%) to determine the presence of target protein. The induced protein was initially 34kDa, which it consistent with the previous research (Andersson, Marlene, et al. 2017).
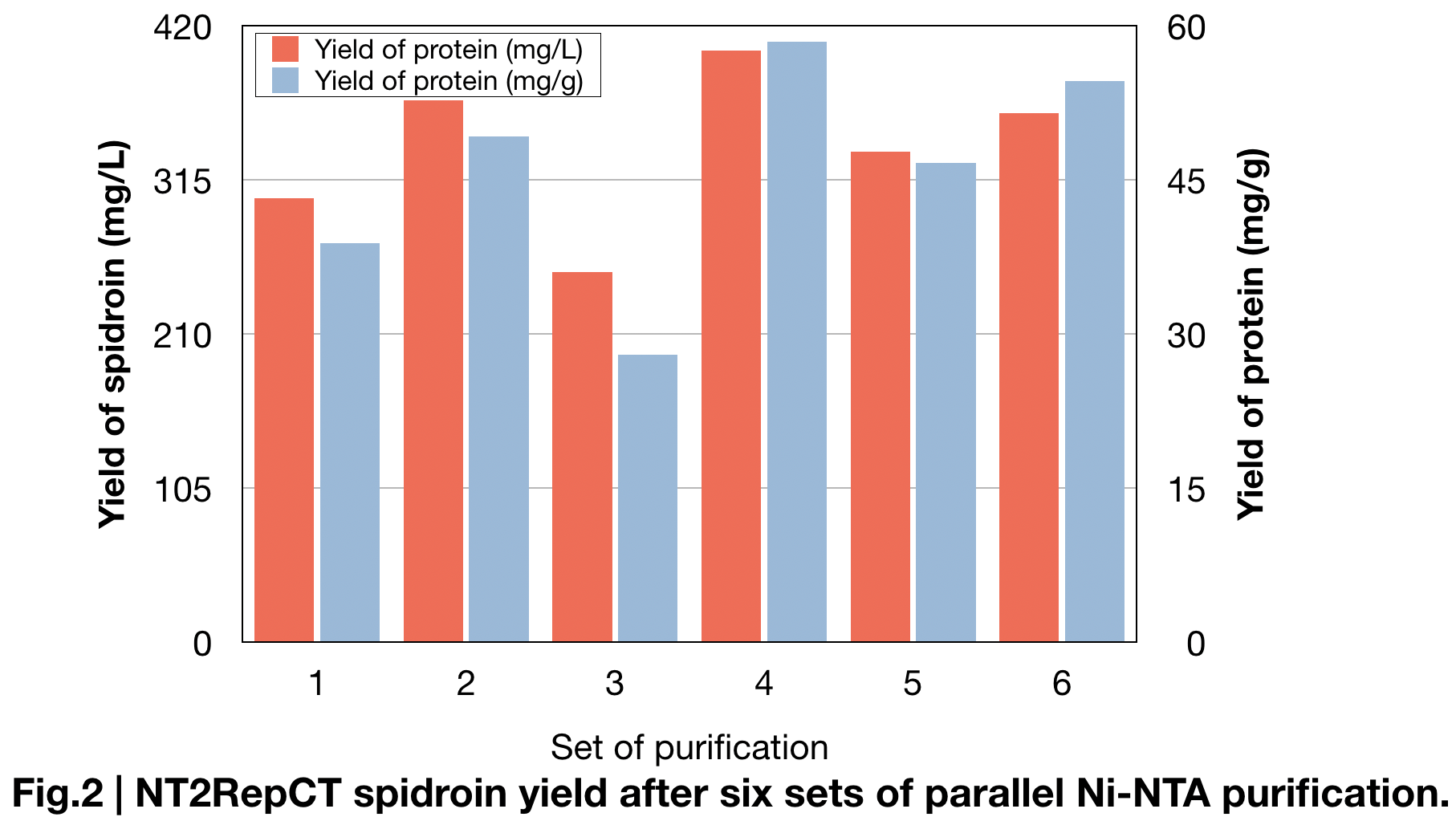
Spidroin Production measure
BCA protein essay kit give us the specified data of the average amount of protein we extracted in parallel sets of Ni-NTA purification (Fig.2). Result shows the amount of spidroin in shaking-flask E.coli cultures and yielded average around 336±49 mg protein/L, and 46±10mg/g, which is more than double of the yield in previous research (125mg protein/L) (Andersson, Marlene, et al. 2017) and most yielded protein among all spidroins we produced this year.
Fiber spinning
NT2RepCT has the feature of pH-dependent assembly, because lowered pH and shearing induce adjusts conformational changes that result in conversion from soluble protein to beta-sheet fibers. At pH>7, NT is in highly soluble state, which lead the spidroin to have high solubility. Due to the lock and trigger mechanism, when pH is lowered in the end of the silk duct, NT forms stable dimers and therefore interconnects the spidroins by applying pulling force alone the fiber via protein chains; CT, at the same time, unfolds to form amyloid-like fibrils that serves as the nucleation seeds of the conversion of the repetitive region (2Rep) into beta-sheet structures. (Andersson, Marlene, et al. 2017)
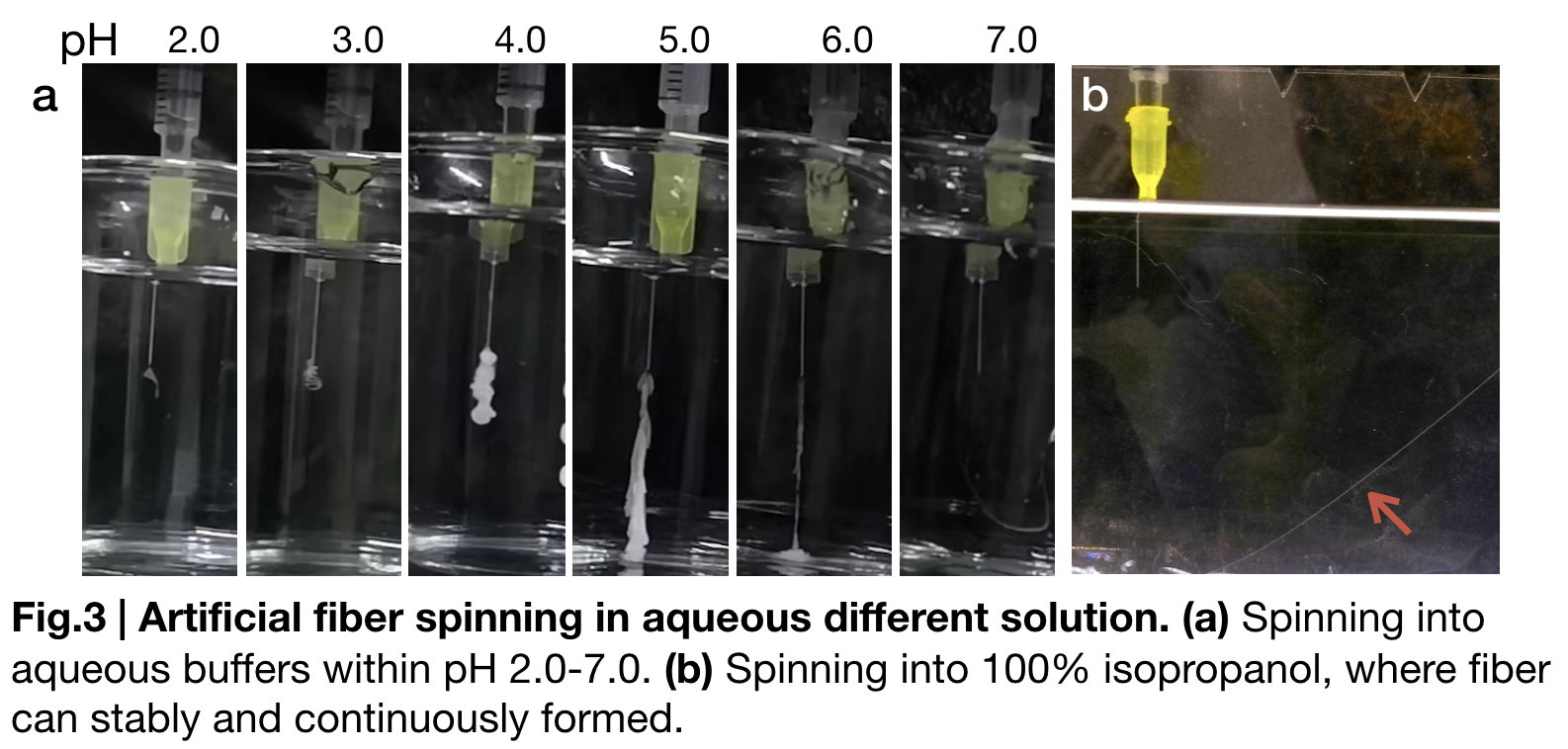
To further substantiate the role of pH in fiber spinning, we carried out artificial fiber spinning in different aqueous buffers that valued from (pH2.0 to pH7.0) with same extruding speed. (Fig.3.a) sketches these relationships. When pH of the buffer bath is from 2.0 to 4.0 gelatinous substance forms and attach to the syringe until disappearing shortly in the solution. Continuous fibers formed when buffer pH is in between 5.0 to 6.0. Fiber is visually continuously formed in pH=7.0 buffer, but disrupted easily when we try to reach the fiber and lift. Besides the pH change, in our biomimetic spinning, we also used 100% isopropanol (Fig.3.b) in the fibre spinning solution and gained a continuous and uniformly-distributed fibre that would not be interrupted by lifting to collect.
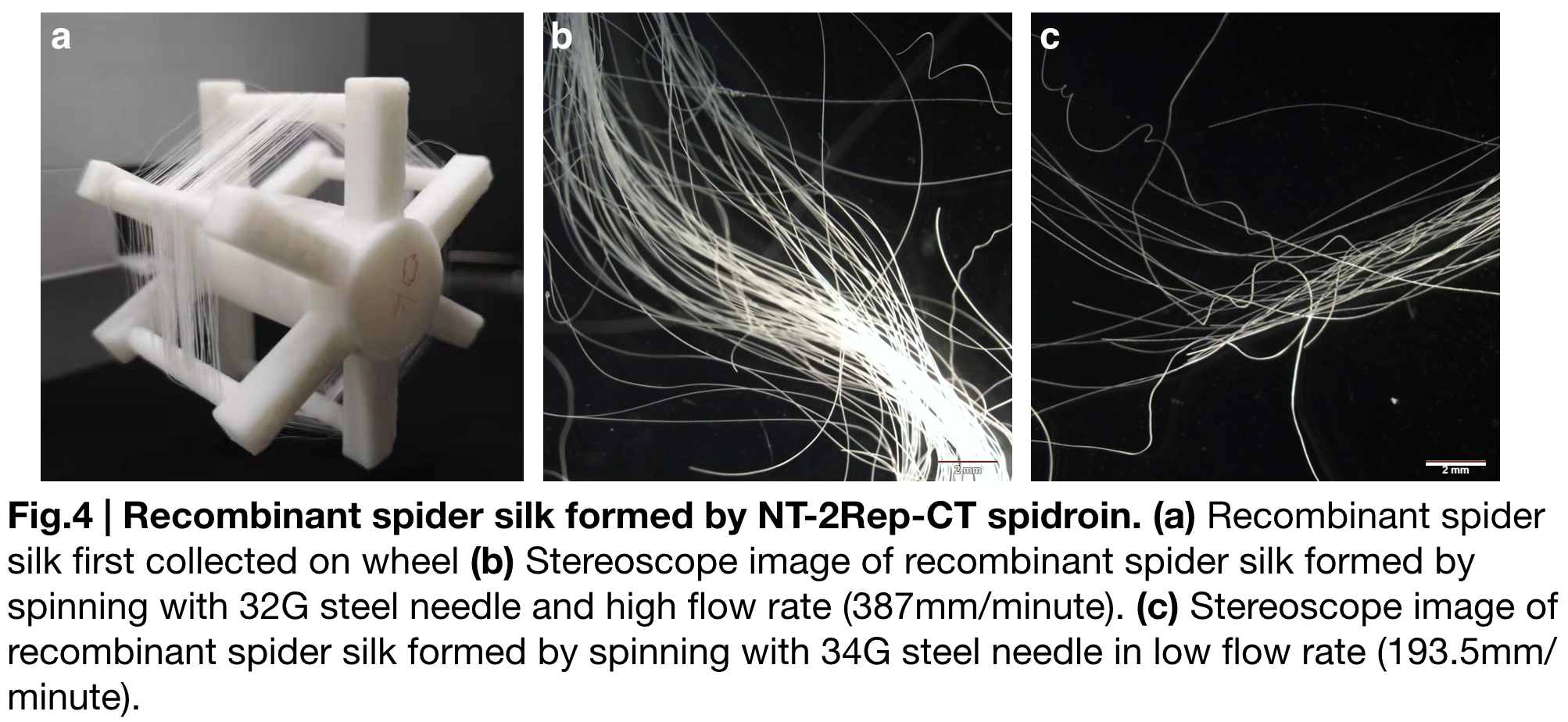
NT-2Rep-CT spidroin formed continuous and stable recombinant silk in our artificial spinning. (Fig.4.a.) To investigate the role of flow rate and tip diameter of the syringe, we artificially spun spidroin NT-2Rep-CT into 100% isopropanol and varied these factors. Stereoscope image was taken for both kind of silk, and showed no difference in their features including diameter or light transmittance. Applying the result to the role of each region of the spidroin, we believe the spider silk property is subjectively caused by solubility of NT, unfolding property of CT and beta-sheet formed by the extensive region Rep.
Scanning electron microscopy of fibers
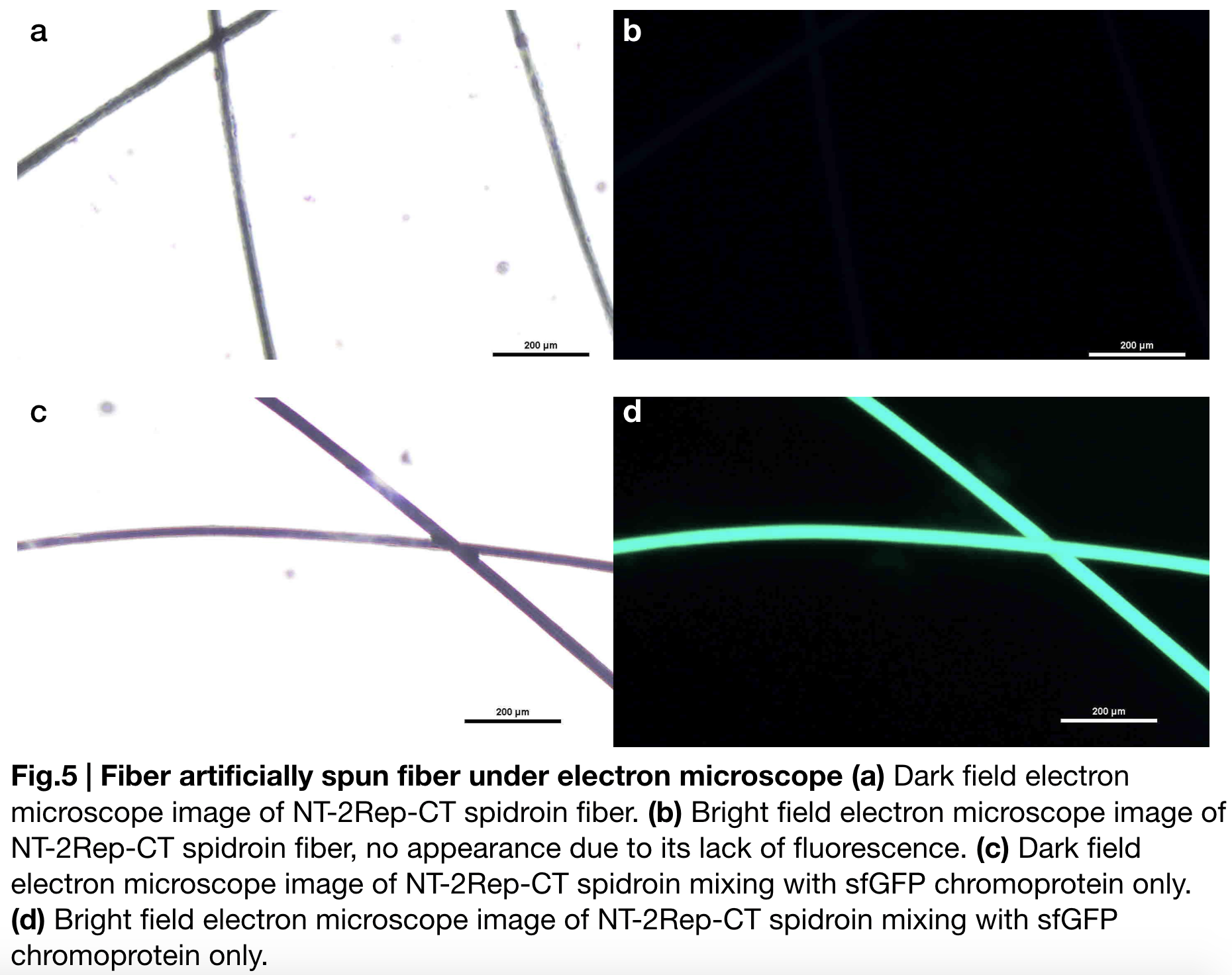
Aiming to verify the distribution uniformity of NT2RepCT after spinning into fiber, we looked at our artificial spun silks by NT-2Rep-CT spidroin and concocting NT-2Rep-CT spidroin with sfGFP chromoprotein under electron microscope (Fig.5), the role of sfGFP chromoprotein here is for us to clearly observe our silk under electron microscope. We randomly selected the cross section in our continuous silk, it is clearly shown that the fiber circularity is qualified as a uniformly distributed silk. Without sfGFP mixed, the spider silk spun by NT-2Rep-CT spidroin only can not be observed from the positive electron microscope image due to its lack of fluorescence.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
| None |